
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
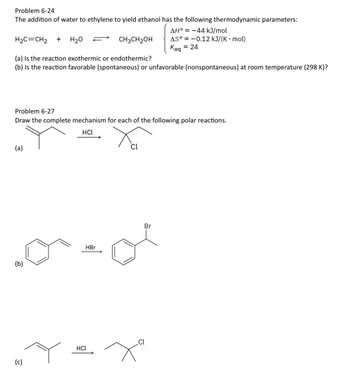
Transcribed Image Text:Problem 6-24
The addition of water to ethylene to yield ethanol has the following thermodynamic parameters:
AH° = -44 kJ/mol
H2C=CH2 +
H2O
CH3CH2OH
AS-0.12 kJ/(K⚫ mol)
Keq = 24
(a) Is the reaction exothermic or endothermic?
(b) Is the reaction favorable (spontaneous) or unfavorable (nonspontaneous) at room temperature (298 K)?
Problem 6-27
Draw the complete mechanism for each of the following polar reactions.
HCL
(a)
(b)
HBr
(c)
HCL
Cl
Br
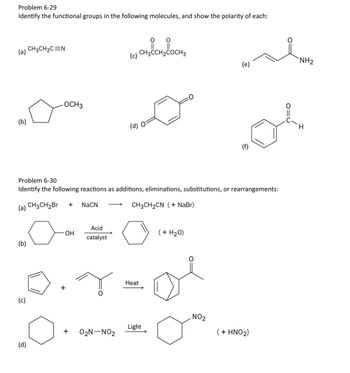
Transcribed Image Text:Problem 6-29
Identify the functional groups in the following molecules, and show the polarity of each:
(a)
CH3CH2C=N
CH, CH, COCH
(c)
CH3CCH2COCH3
NH2
(e)
OCH3
(b)
(d) O
Problem 6-30
Identify the following reactions as additions, eliminations, substitutions, or rearrangements:
(a) CH3CH2Br
+ NaCN
CH3CH2CN ( + NaBr)
Acid
-OH
(+ H2O)
catalyst
(b)
+
(c)
Heat
NO2
Light
+
02N-NO2
(+ HNO2)
(d)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- PROBLEM 3-5 All of the following names are incorrect or incomplete. In each case, draw the structure (or a possible structure) and name it correctly. (a) 2-methylethylpentane (c) 3-dimethylhexane (e) 2-bromo-3-ethylbutane (b) 2-ethyl-3-methylpentane (d) 4-isobutylheptane (f) 2-diethyl-3-methylhexanearrow_forwardPROBLEM 3-11 huon Give IUPAC names for the following compounds: (a) The three isomers of C5H12 (b) (c) CH3 (CH3)2CHCH₂CHCH3 (d) CH3 1 CH3CH₂CHCHCH3 CH3 CH3 (CH3)3CCH₂CH₂CH CH3arrow_forwardPROBLEM 4-30 Rank the following radicals in decreasing order of stability. Classify each as primary, second- ary, or tertiary. (a) The isopentyl radical, (CH3)2CHCH₂-CH₂ (d) (b) The 3-methyl-2-butyl radical, CH3-CH-CH(CH3)2 (c) The 2-methyl-2-butyl radical, CH3-C(CH3)CH₂CH3arrow_forward
- What are the missing reagent(s) for the reaction below? (Select all that apply? OH PBr3 A ☐ A C B D 1) ??? 2) NaCN SOCI₂ NEt3 B TSCI NEt3 с с CN HCN Darrow_forwardPROBLEM 3-4 Provide IUPAC names for the following compounds. (a) (CH3)2CHCH½CH3 (b) CH3— С (СН3)2 — СHз - CH,CH, CH, CH,CH, (c) CH,CH,CH,CH–CH(CH,), (d) CH,-CH-CH,-CH-CH, | Ç(CH,), CH,-CHCH,CH, e) CH,CH,CHCHCH, (f) (CH,),C-CH–CH,CH,CH, CH(CH,),arrow_forwardPROBLEM 3-3 Write structures for the following compounds. (a) 3-ethyl-4-methylhexane (b) (d) (c) 4-tert-butyl-2-methylheptane 3-ethyl-5-isobutyl-3-methylnonane 5-isopropyl-3,3,4-trimethyloctanearrow_forward
- PROBLEM 3-16 Which of the following cycloalkanes are capable of geometric (cis-trans) isomerism? Draw the cis and trans isomers. (a) 3-ethyl-1,1-dimethylcyclohexane (c) 1-ethyl-3-methylcyclopentane (b) 1-ethyl-3-methylcycloheptane (d) 1-cyclopropyl-2-methylcyclohexanearrow_forwardPROBLEM 3-31 Name the following compounds. (b) O (d)arrow_forwardPROBLEM 1-9 Draw the important resonance forms of the following cations and anions: (a) (b) (c) (e) + CH3O–CH–CH=CH–NH NH₂ + OH H₂C=CH-C=OH H3C-C-CH-CH=CH-CN (d) H H₂N-CH=CH-C-OCH3 H3C-CH-C-CH=CH-CN NH || H₂C=CH-C-CH₂arrow_forward
- PROBLEM 3-2 Name the following alkanes and haloalkanes. When two or more substituents are present, list them in alphabetical order. (a) CH₂ CH3 CH₂-CH-CH₂-CH₂ (Sd-592) Ivjud-riboser adi lo comes T soitujzdus ivalla to sergab snt no boand sas aquorg rivud-1 Br CH₂CH3 POTCH3-CH-CH-CH3 nothing (b) (2) cimbrogaz (c) CH–CH, CH,CH(CH,), 1 Todll trong (cruiol sda ba H I TOLE asilo sao CH₂-CH₂-CH-CH-CH₂-CH₂-CH₂ (d) CH₂-CH-CH₂ CH₂ CH₂ T CH-CH₂-CH₂-CH₂-CH₂-CH₂-CH-CH-CH₂arrow_forwardPROBLEM 4-25 In the presence of a small amount of bromine, cyclohexene undergoes the following light- promoted reaction: + trace Br₂ hv cyclohexene (a) Propose a mechanism for this reaction. (b) Draw the structure of the rate-limiting transition state. Br 3-bromocyclohexene + HBrarrow_forwardPROBLEM 11-38 Develop syntheses for the following compounds. As starting materials, you may use cyclopentanol, alcohols containing no more than four carbon atoms, and any common reagents and solvents. (a) trans-cyclopentane-1,2-diol (b) 1-chloro-1-ethyleyclopentane f (c) OCH, CH₂ (e) OCH₂CH₂ C-CH₂CH₂ (1) OCH,arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning