
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
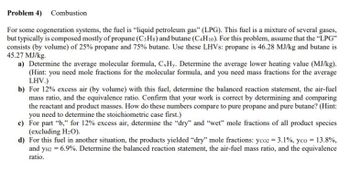
Transcribed Image Text:Problem 4) Combustion
For some cogeneration systems, the fuel is "liquid petroleum gas" (LPG). This fuel is a mixture of several gases,
but typically is composed mostly of propane (C3Hs) and butane (C4H10). For this problem, assume that the "LPG"
consists (by volume) of 25% propane and 75% butane. Use these LHVs: propane is 46.28 MJ/kg and butane is
45.27 MJ/kg.
a) Determine the average molecular formula, CxHy. Determine the average lower heating value (MJ/kg).
(Hint: you need mole fractions for the molecular formula, and you need mass fractions for the average
LHV.)
b) For 12% excess air (by volume) with this fuel, determine the balanced reaction statement, the air-fuel
mass ratio, and the equivalence ratio. Confirm that your work is correct by determining and comparing
the reactant and product masses. How do these numbers compare to pure propane and pure butane? (Hint:
you need to determine the stoichiometric case first.)
c)
For part "b," for 12% excess air, determine the "dry" and "wet" mole fractions of all product species
(excluding H₂O).
d) For this fuel in another situation, the products yielded "dry" mole fractions: yco2 = 3.1%, yco = 13.8%,
and yH2 = 6.9%. Determine the balanced reaction statement, the air-fuel mass ratio, and the equivalence
ratio.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 9 steps

Knowledge Booster
Similar questions
- Demonstrate how enthalpies 1, 2, and 3 were determined.arrow_forwardPlease correct answer and don't use hand ratingarrow_forwardalculate the change in the enthalpy of3.5 moles of Kryptonite (KyO7) in gas phase whenit is heated from 50°Cto 120°CatP= 3.7 atm.The associated molar heat capacity at constantpressure satisfies̄Cp=a0+b1T. The value ofthe coefficients are:a0= 25.503 J K−1mol−1,b1= 13.612×10−3J K−2mol−1arrow_forward
- All parts plzarrow_forwardYou have a calorimeter with 100 g of water at 22.6ᵒC and a 50.0 g piece of copper that you have heated to 98.7ᵒC. You place the copper in the water and wait for the temperature of the water to stop rising. What will be the highest temperature of the water? (Specific heats: water = 4.184 J/gᵒC, copper = 0.385 J/gᵒC )arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The