Question
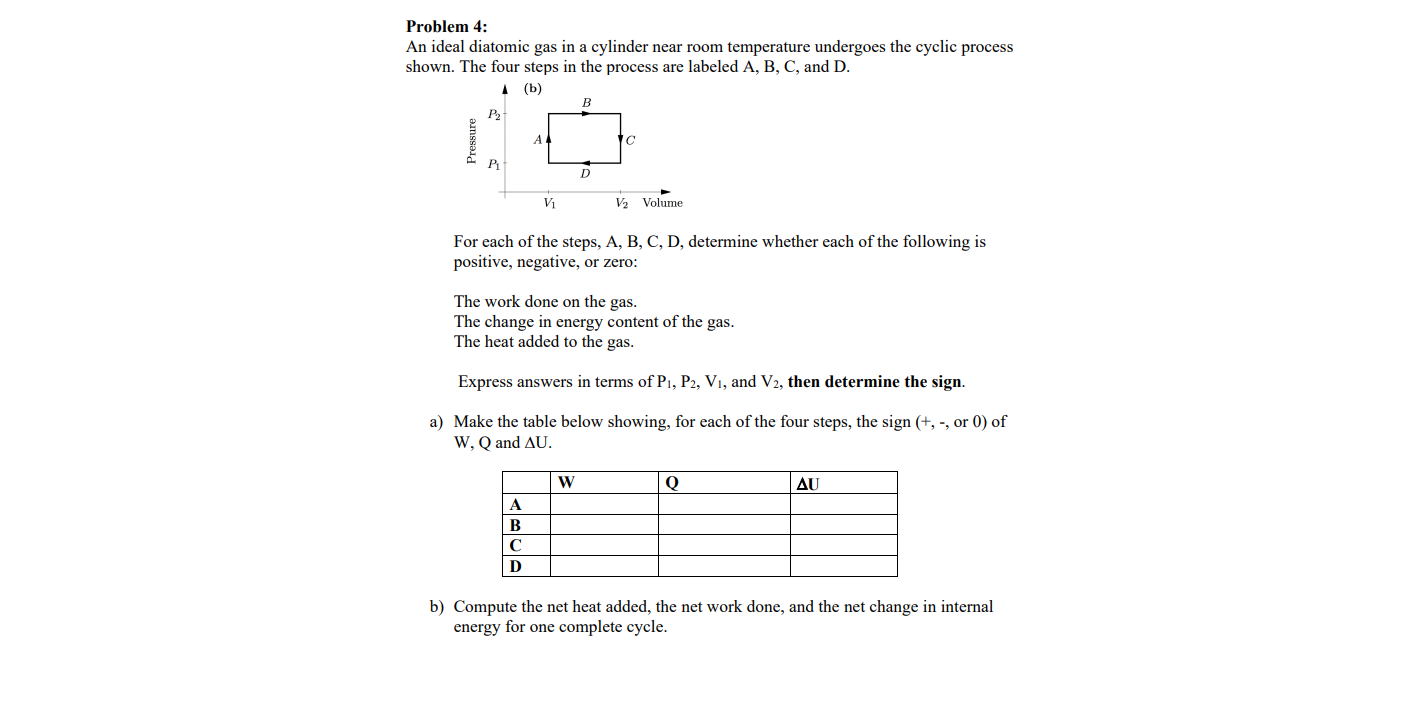
Transcribed Image Text:Problem 4:
An ideal diatomic gas in a cylinder near room temperature undergoes the cyclic process
shown. The four steps in the process are labeled A, B, C, and D.
1 (b)
P2
V2 Volume
Vị
For each of the steps, A, B, C, D, determine whether each of the following is
positive, negative, or zero:
The work done on the gas.
The change in energy content of the gas.
The heat added to the gas.
Express answers in terms of P1, P2, V1, and V2, then determine the sign.
a) Make the table below showing, for each of the four steps, the sign (+, -, or 0) of
W, Q and AU.
AU
B
D
b) Compute the net heat added, the net work done, and the net change in internal
energy for one complete cycle.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 7 steps with 7 images

Knowledge Booster
Similar questions
- A 2100 cm³ container holds 0.10 mol of helium gas at 350°C. Part A How much work must be done to compress the gas to 1200 Cm² at constant pressure? Express your answer with the appropriate units. ▸ View Available Hint(s) W = 220 J ✓ Correct Part B Previous Answers How much work must be done to compress the gas to 1200 Cm³ at constant temperature? Express your answer with the appropriate units. ► View Available Hint(s) W = Submit μA Value Previous Answers Units ? X Incorrect; Try Again; 4 attempts remainingarrow_forwardA cylinder contains 0.250 mol of carbon dioxide (CO₂) gas at a temperature of 27.0°C. The cylinder is provided with a frictionless piston, which maintains a constant pressure of 1.00 atm on the gas. The gas is heated until its temperature increases to 127.0°C. Assume that the CO₂ may be treated as an ideal gas. Part A How much work is done by the gas in this process? Express your answer in joules. — ΑΣΦ W = Submit Part B On what is this work done? The work is done on the piston. The work is done on the cylinder. Submit Part C Request Answ AU = What is the change in internal energy of the gas? Express your answer in joules. ΑΣΦ Submit Request Answer Request Answer P Pearson W ? ? J Jarrow_forwardFor a planet to have an atmosphere, gravity must be sufficient to keep the gas from escaping The escape speed a particle needs to escape the earth's gravitational attraction is 1.1 x 10 m/s. The motion of projectiles never depends on mass, so this escape speed applies equally to rockets and to molecules in the earth's upper atmosphere. Part A At what temperature does the rms speed of nitrogen molecules equal the escape speed? Express your answer in kelvins. ΜΕ ΑΣΦΑ T- Submit Part B T- Request An At what temperature does the mms speed of hydrogen molecules equal the escape speed? Express your answer in kelvins. VAZO Submit ? K Karrow_forward
- The thermodynamic cycle of a heat engine using ideal helium gas is shown in the P-V diagram. The ratio of heat capacities y (also called ratio of specific heats) is the ratio of the heat capacity at constant pressure Cp to the heat capacity at constant volume Cy. For ideal helium gas, y = 1.67. Select the stage that is paired correctly with the process it represents in the P-V diagram. 1-2: adiabatic process O2-3: isochoric process 3-4: isothermal process 4-1: isobaric process p (kPa) 0.8 0.6- 0.4- 0.2 0.0 2 0.2 0.4 0.6 1 0.8 V(m³) 1.0arrow_forwardI Rev Part A How much work is done by the gas in the process shown in the figure? (Figure 1) Express your answer in joules. V AZO ? W = J Submit Request Answer Figure 1 of 1 > Provide Feedback Р (КРа) 400- 200 i V (cm³) 100 200 300arrow_forwardIn an engine, an almost ideal gas is compressed adiabatically to half its volume. In doing so, 2710 J of work is done on the gas. Part A ▼ How much heat flows into or out of the gas? Express your answer with the appropriate units. ? Q = Value Units Submit Request Answer Part B What is the change in internal energy of the gas? Express your answer with the appropriate units. HẢ ? AU = Value Units Submit Request Answerarrow_forward
- An ideal gas at 20°C consists of 2.2 x 1022 atoms. 3.2 J of thermal energy are removed from the gas. Part A What is the new temperature in °C? Express your answer in degrees Celsius. DA ΑΣφ T = °C Submit Request Answerarrow_forwardI need solutions question Barrow_forwardA cylinder contains 0.250 mol of carbon dioxide (CO₂) gas at a temperature of 27.0°C. The cylinder is provided with a frictionless piston, which maintains a constant pressure of 1.00 atm on the gas. The gas is heated until its temperature increases to 127.0°C. Assume that the CO₂ may be treated as an ideal gas. Express your answer in joules. AU = Submit Part D Submit How much heat was supplied to the gas? Express your answer in joules. Part E W₁ = ΑΣΦ | Submit Request Answer ΑΣΦ Request Answer Request Answer < Return to Assignment Provide Feedback www How much work would have been done if the pressure had been 0.50 atm? Express your answer in joules. VE ΑΣΦ P Pearson = ? ? J ? J Jarrow_forward
arrow_back_ios
arrow_forward_ios