
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
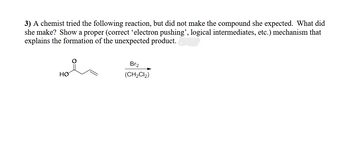
Transcribed Image Text:3) A chemist tried the following reaction, but did not make the compound she expected. What did
she make? Show a proper (correct ‘electron pushing”, logical intermediates, etc.) mechanism that
explains the formation of the unexpected product.
HO
Br2
(CH2Cl2)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- 5) Provide the expected mechanism for the following reaction. Please show all intermediates and use arrows to show the movement of electrons (you do not have to show transition states). (1 pt) ii CI NaOH (2 equiv)arrow_forwardPredict the product(s) of the following reactions, including stereochemistry when necessary and identify the mechanism of each substitution reaction (SN1 vs SN2). Draw the reaction mechanism (reaction arrows) for any one of the reactions to show how the product is formed.arrow_forwardWhat are the necessary reagents for the below transformations to occur? Provide a mechanism for b) (the amount of arrows do not indicate the specific number of steps required) a) b) OH 111 CO,CH, CO₂CH3 om H M SO OHarrow_forward
- Draw an arrow-pushing mechanism and identify the product(s) for each reaction below and specify the most likely mechanism by which the product(s) is formed (SN1, SN2, El, E2, or a combination of these.) Br NACN DMF Br NaOCH3 CH;OH (ii) HCI OHarrow_forwardGive the product of the following reaction. NO, HI NO, NO, он NO There is no reaction under these conditions or the correct product is not listed. NO2 NO,arrow_forwardMethionine, C5H₁1NO2S, is an essential amino acid in humans that is important in angiogenesis and the growth of new blood vessels. When methionine is burned in the presence of excess oxygen, O2, what products are formed? Select all products formed. NO₂ S₂ CO₂ No answer text provided. N₂ H₂O SO2arrow_forward
- Do not give handwriting solution.arrow_forwardCan someone pleae help me with this question? The question is: Draw a reasonable mechanism for this reaction. All compounds involved in each stage of the mechanism must be enclosed in a box, and each box must be connected to the next one with a straight arrow. Use electron-flow arrows to show the movement of electrons in each step.arrow_forwardFill in the boxes with the missing products of each reaction. For box B to C, suggest a mechanism for that reaction step. EtO₂C CO₂Et NaOEt EtOH NaOEt EtOH Br H3O+ A Barrow_forward
- Predict the major products for the following reactions. Pay careful attention when orientation is a factor. Draw just one major product, the full mechanism to form it (draw arrows and electrons) in each case and define which reaction (name it) was carried out: NaOH Cl₂ CH₂OH H₂O H₂SO, cat Br HCI 1. BH₂ THF 2. H₂O₂, H₂O, NaOHarrow_forwardYou attempt to synthesize ethyl isopropyl ether using the following scheme, but you find that you are getting a large amount of some minor product. Br NaOCH2CH3 HOCH,CH3 a) What is the side product you are forming and what would be a better way to synthesize ethyl isopropyl ether? b) What would be the major product of the following reaction? Br NaOCH,CH3 HOCH,CH3arrow_forwardOn acid-catalyzed dehydration, 1-butanol (CH3CH2CH2CH2OH) can be converted to 1-butene. Write out an equation for the reaction Assign each the appropriate symbol for the mechanism of the reaction (E1 or E2) Draw a suitable mechanism for the reactionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
