
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chemistry
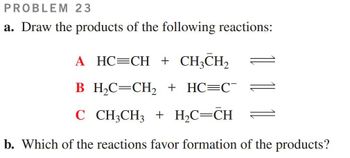
Transcribed Image Text:PROBLEM 23
a. Draw the products of the following reactions:
A HC=CH + CH3CH2
B_H₂C=CH2 + HC=C¯
C CH3CH3 + H₂C=CH
b. Which of the reactions favor formation of the products?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- The following reaction has two possible products A and B. Which is the major product formed? H H H/Pt + A B Link O A Ов O a 50/50 mixture of A and B is formed Ill..arrow_forwardNonearrow_forwardIdentify the best reagents to complete the following reaction. 1. NH2OH, TSOH 2. H2SO4 ū NH₂ ✔ Q Problem 29 of 40 A B с E 1. NaN3 2. CH3CH2OH, heat 1. NH2OH, TSOH 2. H2SO4 1. NaN3 2. H₂O, heat mCPBA NaOH, Br2 Done Submitarrow_forward
- Off Pd (ally) ₂ Cl₂ (S)-BINAP лос. сами UTIPS ال A Licl H₂O, DMSO 4 1) B₂-C1 2) ивиц NF 3) MsC1 4) Na I B 3 eg DIBAL -OH -OTTPSarrow_forwardThe pKa of p-cyclopropylbenzoic acid is 4.45. Is cyclopropylbenzene likely to be more reactive or less reactive than benzene toward electrophilic bromination? Explain.arrow_forwardА9. Which of the following statements is true for an Sn2 reaction? The rate of an SN2 reaction depends on the concentration of: A the electrophile only В the nucleophile the product D the nucleophile and electrophilearrow_forward
- Problem 65 of 80 Submit Draw the product of the reaction shown below. Ignore inorganic byproducts. Br2 (1 equiv) FeBrз Select to Draw > Qarrow_forwardAnswer the following questions based on the given reaction scheme: A OH он So;Na conc. H,SO, NaOH ŠO,Na Br C он OH он Br acid Br SOgNa Br 200 °C H,SO, SO,Na Which step(s) require heating? DA OB OC OD OE In which step(s) does aromatic halogenation occur? DA OB OC DE In which step(s) does aromatic sulfonation occur? DA OB OC OD OE What is the name of the product?arrow_forwardplease choose correct answer for 20arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
