
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:!
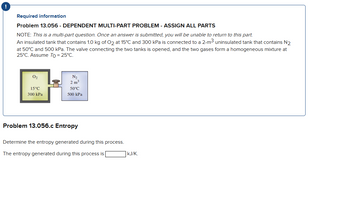
Required information
Problem 13.056 - DEPENDENT MULTI-PART PROBLEM - ASSIGN ALL PARTS
NOTE: This is a multi-part question. Once an answer is submitted, you will be unable to return to this part.
An insulated tank that contains 1.0 kg of O2 at 15°C and 300 kPa is connected to a 2-m³ uninsulated tank that contains N2
at 50°C and 500 kPa. The valve connecting the two tanks is opened, and the two gases form a homogeneous mixture at
25°C. Assume To = 25°C.
0₂
15°C
300 kPa
11
N₂
2 m³
50°C
500 kPa
Problem 13.056.c Entropy
Determine the entropy generated during this process.
The entropy generated during this process is
KJ/K.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 8 steps with 8 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Steam at a rate of 200 kg/min enters a turbine at 350°C and 40 bar through a 7.5-cm internal diameter pipe. The turbine operation is adiabatic, and the effluent leaves as saturated water at 5 bar through a 5-cm diameter pipe. 1. Calculate the work produced by the turbine in kW. 2. What is the enthalpyand phase of the effluent stream? 21 If it leaves the turbine at 75C and 5 bar 22 If it leaves the turbine at 30C and 5 bar 7.5arrow_forwardA and B containers (fixed volume) are connected together by a valve. Tank A contains 400 kPa and 0.3 m3 refrigerant 134a at 60% dryness. Tank B contains 0.5 m3 of refrigerant 134a at 240 kPa pressure and 100 oC temperature. Then the valve is opened and the system reaches 280 kPa equilibrium pressure. Calculate the final temperature.arrow_forwardParvinbhaiarrow_forward
- 303 nt inces Required Information NOTE: This is a multi-part question. Once an answer is submitted, you will be unable to return to this part. Steam flows steadily through an adiabatic turbine. The inlet conditions of the steam are 4 MPa, 500°C, and 80 m/s and the exit conditions are 30 kPa, 92 percent quality, and 50 m/s. The mass flow rate of the steam is 13 kg/s. The properties of the steam are v=0.086442 m³/kg, h = 3446 kJ/kg, and 2 = 2437.7 kJ/kg. P = 4 MPa 7-500C VVER Steam P₂ = 30 kPa 4y=0.92 V₂50 m/s Determine the turbine inlet area. The turbine inlet area is m².arrow_forwardAs shown in the figure, a 320-ft³ tank contains 25 lb of H₂O initially at 30 lbf/in². The tank is connected to a large steam line carrying steam at 200 lbf/in², 450°F. Steam flows into the tank through a valve until the tank pressure reaches p2 = 100 lbf/in² and the temperature is 400°F, at which time the valve is closed. Step 1 Steam at 200 lbf/in.2, 450°F Am 12 = i (1) Valve AD Determine the amount of mass that enters the tank, in lb. lb (1) Determine the amount of mass that enters the tank, in lb, and the heat transfer to the tank from its surroundings, in Btu. Tank Initially: 30 lbf/in.², m₁ = 25 lb (2) Finally: P₂ lbf/in.², 400°F.arrow_forwardD Question 1 Use this figure for Questions 1 to 5. A hot-water stream at 90 °℃ enters an insulated mixing chamber with a mass flow rate of mh=0.75 kg/s where it is mixed with a stream of cold water at 25°℃ in a steady state steady flow process. It is desired that the mixture leaves the chamber at 45°℃ as shown in the Figure Hot water P₁=300 kPa T₁=90°C m=0.75 kg/s Cold water P-300 kPa T: 25% m=? 1 2 Mixing Chamber Mixed warm water Ps-300 kPa T=45°C m3 For Question 1: The specific enthalpy of the hot water, h1, in kJ/kg isarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY