
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please help me with all 3 parts
Transcribed Image Text:Press
esc to exit full screen
< Question 29 of 29
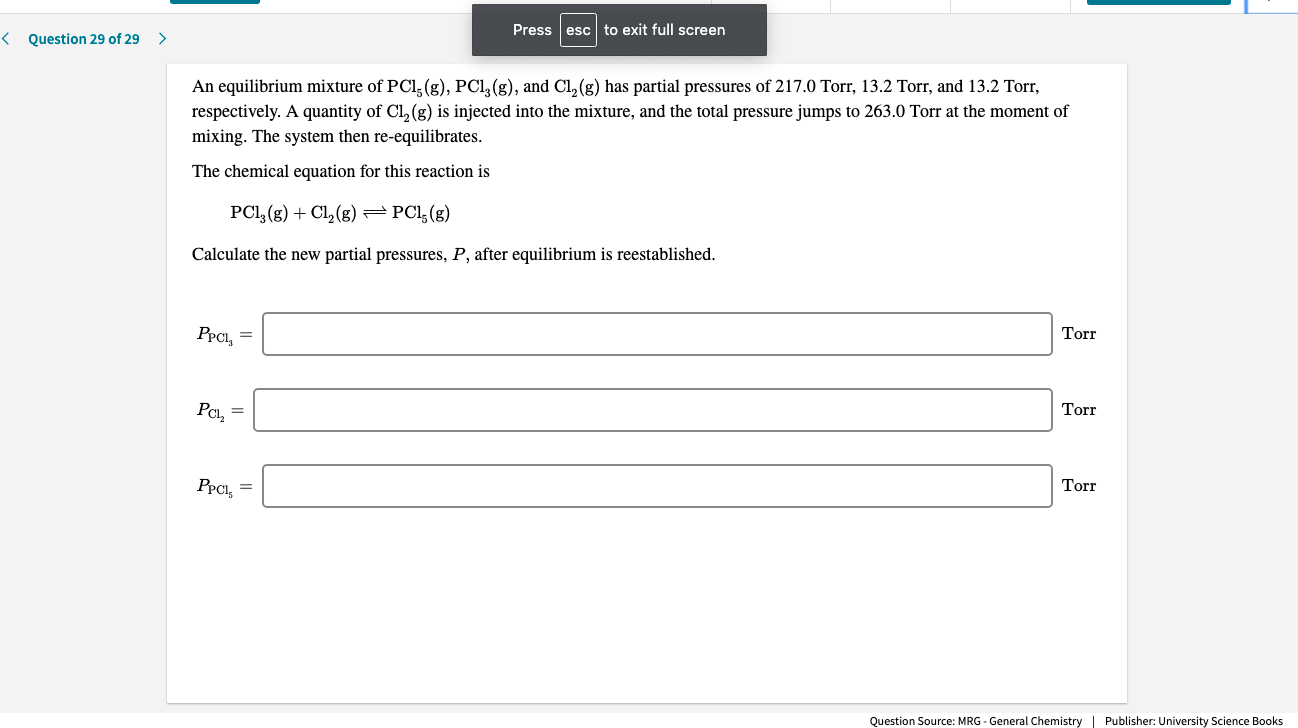
An equilibrium mixture of PCl, (g), PCI, (g), and Cl, (g) has partial pressures of 217.0 Torr, 13.2 Torr, and 13.2 Torr,
respectively. A quantity of Cl, (g) is injected into the mixture, and the total pressure jumps to 263.0 Torr at the moment of
mixing. The system then re-equilibrates.
The chemical equation for this reaction is
PCI, (g) + Cl, (g) = PCl;(g)
Calculate the new partial pressures, P, after equilibrium is reestablished.
PPCI, =
Torr
PCl, =
Torr
PPCI, =
Torr
Question Source: MRG - General Chemistry | Publisher: University Science Books
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 8 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Br [A] fins [B] Br [C] 17. Discuss the relationship between Compounds A, B and C. Then draw a diagram to show what you would expect to see on a TLC plate if you compared the three compounds on the same plate. 120m 9marrow_forwardWhy is Cp > Cv?arrow_forwardI do need help with this problem. It is multiparty. Thank you!arrow_forward
- Phase change properties of pure substances Cu C6H12 C (C6H5CH₂)₂0 (CH3CH₂)20 CH3CH₂OH (CH₂OH)2 NH₂COH Au C C6H14 H₂ copper cyclohexane diamond dibenzyl ether diethyl ether ethanol ethylene glycol formamide gold graphite hexane hydrogen 1084.62 6.7 4440 1.8 -116.22 -114.14 -13.0 2.57 1064.18 4489 -95.27 -259.16 2560 80.7 298 34.4 78.24 197.5 217 2836 3825 68.72 -252.879 0.385 1.841 0.51 2.369 2.438 2.394 2.389 0.129 0.709 2.27 14.304 13.26 2.68 -- 7.19 4.931 9.96 8.44 12.55 117.4 13.08 0.12 29.97 45.6 26.52 38.56 50.5 60.2 324 28.85 0.9 280.3 193.7 242 446 -- 234.4 -240.212 40.2 35.9 61.7 80 29.9 12.69arrow_forwardProblem: If the diameter of a bicycle wheel is 622 mm, what is the diameter of the wheel in inches? (1 inch = 25.4 mm) Solution: 622 mm x 1 inch/25.4 mm = 24.5 inches. Make sure to show all the units in your solutions, as seen in the example above.arrow_forwardDraw the main components that will be present in any HPLC and GC instrument respectively.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY