
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
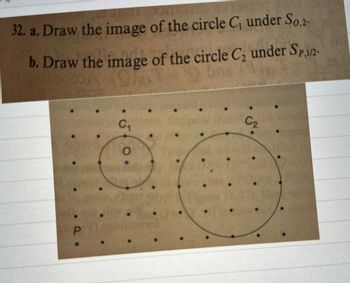
Transcribed Image Text:32. a. Draw the image of the circle C₁ under So,2-
b. Draw the image of the circle C, under SP,1/2-
C₁
02
Pool
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- 11-13arrow_forwardl2 p2 ANSWER ONLYarrow_forwardIndicate whether the following follow the Aufbau Principle 1s2 25? 2p6 35? 3p6 3d10 45? 1s2 252 2p6 3s2 3p6 4s2 3d10 1s2 25? 2p6 35? 3p6 45² 3d10 4p6 55? 4d10 5p6 6s? 5d10 1s2 25? 2p6 3s2 3p6 4s2 3d10 4p6 5s² 4d10 5p6 6s? 4f10 а. yes b. noarrow_forward
- Name the compunds and identify the RS configurationarrow_forwardFollowing the Woodward-Fieser's rules, determine the Amax for the following molecule: X 382 nm 356 nm 378 nm 371 nm Oarrow_forward101 Chem101 C Chegg Search X b My Questions | bartleby X G Identify the LUMO in the MO di x G LUMO in the MO diagram of NC X + -> app.101edu.co M Apps G M Gmail YouTube Maps a AMAZON Translate Gflights USCIS Ь ВАТERBLY C CHEGG > KATAPULK CUBA SUPERMARKET23 Essay Writing Ser... G calculator - Googl... Reading List Question 5 of 22 Submit Identify the LUMO in the MO diagram of NO (shown below). A) o (2s orbitals) B) o (2pz orbitals) Op Tp C) TT (2px and 2py) D) T* (2px or 2py) E) o* (2pz) +arrow_forward
- 21. Which of the following does have the lowest polarizability? Z of Br = 35, I = 53, F = 9, S = 16 Group of answer choices a. F b. I- c. S- d. Br-arrow_forwardDraw a pz orbital and a dx2-y2 orbital.arrow_forwardWhich of the following functions can be normalized (-∞0 ≤x≤∞, a > 0) a. sin (ax) b.cos(ax) e-x²arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY