
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
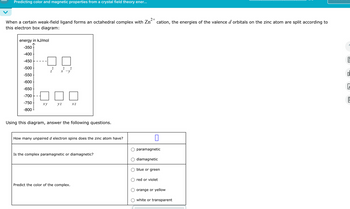
Transcribed Image Text:Predicting color and magnetic properties from a crystal field theory ener...
2+
When a certain weak-field ligand forms an octahedral complex with Zn cation, the energies of the valence d orbitals on the zinc atom are split according to
this electron box diagram:
energy in kJ/mol
-350
-400
-450
-500
2
2 2
Z
x-y
-550
-600
-650
-700
-750
xy
yz
XZ
-800
Using this diagram, answer the following questions.
How many unpaired d electron spins does the zinc atom have?
Is the complex paramagnetic or diamagnetic?
Predict the color of the complex.
☐
paramagnetic
diamagnetic
blue or green
red or violet
orange or yellow
white or transparent
OL
E
9
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Match the orbitals below to the higher and lower energy levels when an octahedral complex undergoes ligand field splitting. dx2-y2 Orbital 1 Orbital 1 Orbital 2 Orbital 3 Orbital 4 Orbital 5 L d₂2 Orbital 2 L dyz Orbital 3 dxz Orbital 4 [Choose] ◊ [Choose] ↑ [Choose] ◊ [Choose] [Choose] ↑ L dxy Orbital 5arrow_forward1. The complex ion [Ni(en)2]2+ is…A. is diamagnetic because all electrons are pairedB. is paramagnetic because all electrons are pairedC. is paramagnetic due to the presence of 2 unpaired electronsD. is diamagnetic due to the presence of 2 paired electrons 50. The complex compound which has the most inert properties is…. A. [PF6]-B. [SF6]C. [SiF6]2-D. [AlF6]3-arrow_forwardoving to another question will save this response. complex [Co(NH3)6l absorbs light at a wavelength of 465 nm. What is the ligand field aplitting energy for the cormplez in Jlaole? 275 x 10 to negative x AMoving to another question will save this response.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY