
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
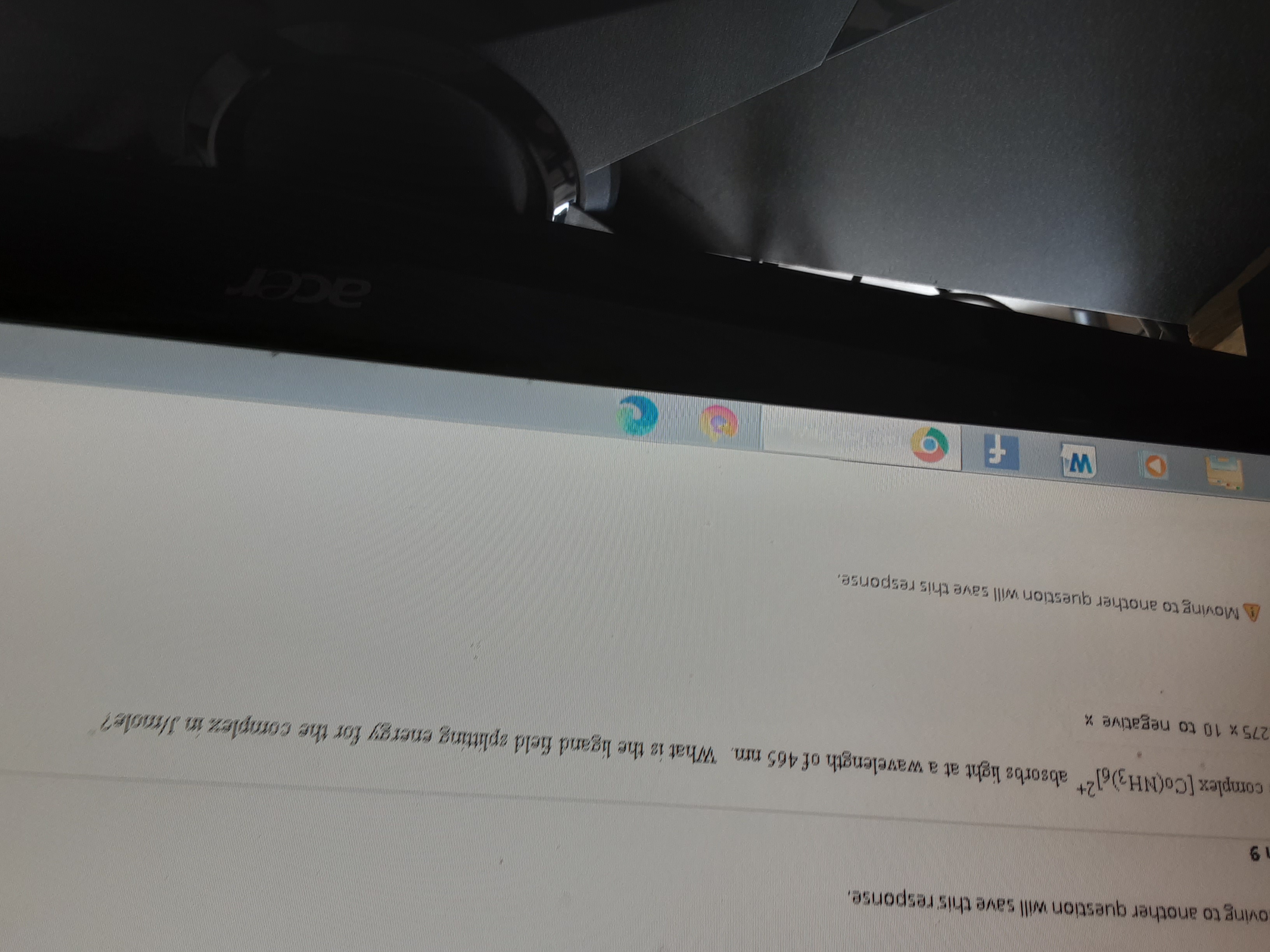
Transcribed Image Text:oving to another question will save this response.
complex [Co(NH3)6l absorbs light at a wavelength of 465 nm. What is the ligand field aplitting energy for the cormplez in Jlaole?
275 x 10 to negative x
AMoving to another question will save this response.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Predicting color and magnetic properties from a crystal field theory ener... 0/5 Izabella 2+ When a certain strong-field ligand forms an octahedral complex with Co²+ cation, the energies of the valence d orbitals on the cobalt atom are split according to this electron box diagram: energy in kJ/mol -300 -350 -400 2 2 2 -450 Z x-y -500 -550 -600 -650 -700 xy yz XZ -750 Using this diagram, answer the following questions. How many unpaired d electron spins does the cobalt atom have? Is the complex paramagnetic or diamagnetic? Predict the color of the complex. paramagnetic diamagnetic blue or green red or violet orange or yellow white or transparent POO 18 Ararrow_forwardThe crystal-field splitting Δ for a given coordination complex is 197 kJ/mol. What is the wavelength of light, in nm, that this complex absorbs?arrow_forwarda) Naz[Fe(CN)4(en)] b) The complex ion of this compound is known to be diamagnetic. Draw the d-orbital splitting diagram for the complex ion. similar to this: dx-y² Aoct { E dry dxz dyz Aet dxz dyz da-y dxy Tetrahedral Octahedral case case dry E E dyz dp- y? Free metal jon Complex Free metal ion Complex M M- (b) (a)arrow_forward
- Predicting color and magnetic properties from a crystal field theory ener... 2+ When a certain strong-field ligand forms an octahedral complex with Rh cation, the energies of the valence d orbitals on the rhodium atom are split according to this electron box diagram: energy in kJ/mol -300 -350 -400 2 2 2 -450 Z x-y -500 -550 -600 -650 -700 xy yz XZ -750 Using this diagram, answer the following questions. How many unpaired d electron spins does the rhodium atom have? Is the complex paramagnetic or diamagnetic? Predict the color of the complex. paramagnetic diamagnetic blue or green red or violet orange or yellow white or transparentarrow_forwardFor [Fe(CN)6]*, please write the valence electron configuration of the metal ion, the ground- state term symbol, determine the number of unpaired electrons, and calculate the ligand field stabilization energy (LFSE) in terms of Ao: 12.arrow_forwardGive the formula of each coordination compound. Include square brackets around the coordination complex. Do not include the oxidation state on the metal. Use parentheses only around polyatomic ligands. a) potassium tetracyanonickelate(II) b) sodium diamminedicarbonatoruthenate(III) c) diamminedichloroplatinum(II)arrow_forward
- 10:58 Search Question 1 of 1 A) [ZnCl₂]²- Which of the following complex ions is paramagnetic? B) [Fe(H₂O)]²+ (H₂O is weak field ligand) C) [Fe(CN)6]4- D) [Co(CN)6]³- E) [Zn(H₂O)]²+ 35 Submit Tap here or pull up for additional resources ]]]arrow_forward3+ When a certain weak-field ligand forms a tetrahedral complex with Mn" according to this electron box diagram: energy in kJ/mol -200 -300 -400 -500 -600 xy yz 2 2 X2 Using this diagram, answer the following questions. How many unpaired d electron spins does the manganese atom. have? Is the complex paramagnetic or diamagnetic? Predict the color of the complex. paramagnetic Odiamagnetic blue or green Ⓒred or violet cation, the energies of the valence d orbitals on the manganese atom are split orange or yellow Owhite or transparentarrow_forwardGive the formula of each coordination compound. Include square brackets around the coordination complex. Do not include the oxidation state on the metal. Use parentheses only around polyatomic ligands. sodium hexachloroplatinate(IV): sodium diamminedicarbonatoruthenate(III): tetraamminediaquairon(III) chloride:arrow_forward
- Determine the electronic configuration (in the form t2g "eg or emt2" , as appropriate) and the ligand field stabilization energy for each of the following complexes (1)[Co(NH3)6] 3* ; (II) [Fe(OH2)6] 2+arrow_forward3. Use ligand field theory to explain why [Ni(CN).]² is diamagnetic while [Ni(CI)4]²- is paramagnetic with a spin state of S = 1.arrow_forward1) Consider the transition metal M which, in neutral form, has an electronic configuration ending in (n-1)d5 ns1. M forms the following complexes: [MCl6]K3, [M(CN)6]K4 and [M(en)3]Cl3. 1a) Fill in the following table Complex ion Oxidation state of M CN Electronic configuration outer shell of M [MCl6]K3 [M(CN)6]K4 [M(en)3]Cl3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY