
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
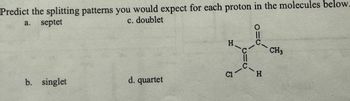
Transcribed Image Text:Predict the splitting patterns you would expect for each proton in the molecules below.
c. doublet
a.
septet
H
CH3
b. singlet
d. quartet
C1
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- 3. Which MO is the LUMO? А В II D IV 8888 I. , II. y, III. y, IV. Y4arrow_forwardUse the following scheme to answer the next three problems. OH HgCl₂ H₂O C 1. BuLi 2. compound D. 3. H HS BF3 SH Earrow_forwardWhich of the following have the S configuration? H3C OH HH Br CH3 ...CI HOOC- H. H. CH3 3. O 2 only O 1 only O 2 and 3 O 1 and 3 2. 1.arrow_forward
- What are the configurations of the two stereogenic carbon atoms in this molecule? CI H 5 6 O 3 2 4 H3C H A. (3R, 6R) B. (3R, 6S) OC. (3S, 6R) OD. (3S, 6S)arrow_forward2. Determine the absolute configuration (R/S) of the molecules below. CI H NH,arrow_forwardPropanal (bp 48°C) and propanol (bp 97°C), both found on Table 4.2, have very similar surfaceareas and dipole moments. Construct an explanation for the large difference in boiling pointsbetween the two.arrow_forward
- Indicate the expected splitting for the protons in the structures like: singlet, doublet, triplet of quartets or other for eachI needed help doing this for A, B, C, and D that are shown in the picture I submited. Can someone help and explain?arrow_forwardWhich molecule has the greatest number of unique protons? How many unique protons does each molecule have?arrow_forwardLI H Br 'к The molecule from question 2 is shown above with an S-configuration. Identify any of the following represenations of th esame molecule that also have the S-configuration. Choose all that apply. 000 FEB 19 Get the mobile app A K B Br LISO Br you с tv S O Finish attempt....arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning