
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
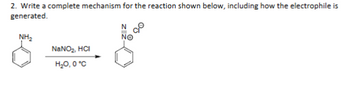
Transcribed Image Text:2. Write a complete mechanism for the reaction shown below, including how the electrophile is
generated.
NH₂
NaNO2, HCI
N
NO
H₂O, 0°C
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- 2. Write a complete mechanism for the reaction shown below, including how the electrophile is generated. HNO3 H2SO4 NO2arrow_forwardAlkyl diazonium salts decompose to form carbocations, which go on to form products of substitution, elimination, and (sometimes) rearrangement. Keeping this in mind, draw a stepwise mechanism that forms all of the following products.arrow_forwardHow does changing the base from −OH to H2O affect the rate of an E2 reaction?arrow_forward
- Rank the following bases in order from slowest E2 reaction rate to fastest. ONa H20 NaOH NH3 OK ONa A В D E Farrow_forwardMajor product and mechanism for this reaction?arrow_forwardRank the attached compounds in order of increasing reactivity in asubstitution reaction with −CN as nucleophile.arrow_forward
- Br CH3OH + Br-Br H3CO The mechanism proceeds through a first cationic intermediate, intermediate 1. Nucleophilic attack leads to intermediate 2, which goes on to form the final product. In cases that involve a negatively charged nucleophile, the attack of the nucleophile leads directly to the product. +Br + CH3OH Br Intermediate 1 Intermediate 2 (product) In a similar fashion, draw intermediate 1 and intermediate 2 (final product) for the following reaction. OH + Br2 + HBr Br racemic mixture • Pay attention to the reactants, they may differ from the examples. In some reactions, one part of the molecule acts as the nucleophile. • You do not have to consider stereochemistry. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate intermediate 1 and intermediate 2 using the → symbol from the dropdown menu.arrow_forwardWrite out the expected splitting pattern for hydrogens Ha, Hp and Hc 3Jab = 10 Hz На Hb 3Jpc = 7 Hz CI Hc Hc Ha:Hb:Hc: doublet, quartet, doublet Ha:Hb:Hc: quartet, quartet, triplet Ha:Hb:Hc: doublet, doublet of triplets, doublet Ha:Hb:Hc: doublet, triplet of doublets, doubletarrow_forwardDraw a detailed, step-wise mechanism for the reaction shown below. SHOW ALL BOND-FORMING AND BOND-BREAKING STEPS. SHOW ALL INTERMEDIATES. 1) NaOH 2) H3O* H3C H3C HOarrow_forward
- Rank the nucleophiles in following group in order of increasing nucleophilicity. H2O, −OH, CH3CO2-arrow_forwardIn EAS bromination reactions, a -CON(CH3)2 substituent on the aromatic ring is: an activator and an o,p-director. a deactivator and a m-director. an activator and a m-director. the substituent catalyzes the 1,2 addition of bromine across the double bond. a deactivator and an o,p-director. Garrow_forwardPredict the major product of the reaction and draw it in the space provided. + HN. NPh NaBH3CN MeOH/ACOHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning