
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question

Transcribed Image Text:Predict the products of the reactions below, and show the mechanism for each reaction
by using curved arrows to indicate the movement of electrons. If there is no reaction,
please write "NR". Note that these problems involve substitution and acid/base reactions.
(a) The pka value of the thiol group (RSH) in this molecule is about 11. THF stands for
tetrahydrofuran and is just the solvent (not involved in the reaction).
HS
Li
Br
in THF
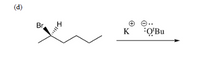
Transcribed Image Text:(d)
Br.
e 0..
K
:Q'Bu
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Give correct detailed Solution with explanation needed with correct answer..don't give Handwritten answer..choose correct answerarrow_forwardII. For the alcohols below, propose at least two syntheses starting from compounds with fewer carbon atoms: OH Fou OH OH Ph OH Pharrow_forward1. Would you expect the following compounds to dissolve in the water layer or 1-hexanol layer, or neither layer? Use a couple sentences to justify each choice you made. (a) KPO. (b) SrSO. (c) FeCl, (d) CH2=CH-CH2-CH3 NO, (e) (f) CH3 0Na® CH H но но HO, O:5=0arrow_forward
- The following sequence of steps converts (R)-2-octanol to (S)-2-octanol. ОН ОН p-TSCI CH,COO Na+ A 1. LIAIH, pyridine DMSO 2. H,О (R)-2-Octanol (S)-2-Octanol Propose structural formulas for intermediates A and B, specify the configuration of each, and account for the inversion of configuration in this sequence.arrow_forwardQuestion 9 of 15 Identify the nucleophile and electrophile in the following acid-base reaction of acetone I and sulfuric acid II shown below. Но S OH + H3C CH3 II A) nucleophile = I; electrophile = || B) electrophile = I; nucleophile = || + H3C CH3arrow_forwardWhat is the major organic product obtained from the following reaction? Br2 CH;COOH, LIBI O (E) 3,4-dibromohexene O (Z) 3,4-dibromohexene O (Z) 1,2-dibromohexene O (E) 1,2-dibromohexenearrow_forward
- Select the organic product(s) of the following reaction below. More than one answer may be selected. 1. CH,CH,MgBr, CH;CH,OCH,CH3 2. HCI, H20 OH OH OHarrow_forwardI would like to see the steps (arrows) that was taking to get the products that is currently displayed. Chemical Reactions and Mechanisms. Dehydration of 4-methyl-2-pentanolarrow_forwardWhat's the correct answer?arrow_forward
- Predict the product(s) for the following reaction, using as much acid and water as necessary.arrow_forwardGive the reagents for the following reaction.arrow_forwardPredict the major products of this organic reaction. c+ С 0: +1 1.03 2. (CH₂)₂S 2. X Click and drag to start drawing a structure.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY