
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Ff.166.
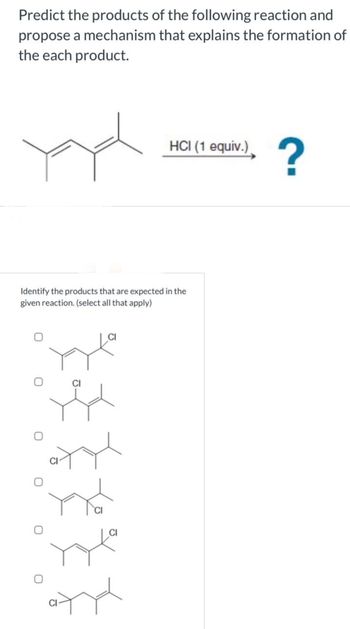
Transcribed Image Text:Predict the products of the following reaction and
propose a mechanism that explains the formation of
the each product.
yad
HCI (1 equiv.)
Identify the products that are expected in the
given reaction. (select all that apply)
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What volume of 1.27 M HCl is required to prepare 197.4 mL of 0.456 MHCI? O a. 70.9 mL O b.5.5 x 10² mL O c.0.0141 mL O d. 3.41 x 10² mL O e. 1.14 × 10² mLarrow_forwardThe accompanying table shows the ages (in years) of 11 children and the numbers of words in their vocabulary. Complete parts (a) through (d) below. Click here to view the data table. Click here to view the table of critical values for the Pearson correlation coefficient. (a) Display the data in a s A. Vocabulary size 3000+ 2400+ 1800- 1200- 600+ 0+ • .. 0 2 4 6 8 Age (years) (b) Calculate the sample r= (Round to three decimal Data Table Age, x 1 OF ANG W SA W N 5 6 3 2 4 6 Print Vocabulary size, y 9 240 540 1100 2300 2600 650 2200 270 1300 2300 Done - X +++ ars) 3000 O D. Vocabulary size 3000+ 2400- 1800- 1200- 600- of 02468 Age (years) Q oarrow_forwardItem 4 4 of 16 I Review I Constants I Periodic Table Gases are different from solids and liquids. In a sample of gas, the molecules are far apart. The gas molecules also move around and collide with each other as well as with the walls of the container. These collisions generate pressure. The pressure of a gas can be measured in different units. One convenient unit of measure is called the atmosphere (atm) because it is based on atmospheric pressure. At sea level, the average pressure is 1 atm. As you get higher in altitude, the pressure steadily drops until you leave the atmosphere, where the pressure is very close to 0 atm. Part C The table below shows the different commonly used units of measuring gas pressure. Use this table in the pressure unit conversions. High-pressure liquid chromatography (HPLC) is a method used in chemistry and biochemistry to purify chemical substances. The pressures used in this procedure range from around 500 kilopascals (500,000 Pa) to about 60,000 kPa…arrow_forward
- 2911/16/-1 {J... macos Monterey... Dashboard scyool Chlorine gas can be prepared in the laboratory by the reaction of hydrochloric acid with manganese (IV) oxide. 4 HCl(aq) + MnO₂ (s) → MnCl₂ (aq) + 2 H₂O(1) + Cl₂(g) A sample of 43.1 g MnO₂ is added to a solution containing 41.3 g HCl. What is the limiting reactant? O MnO₂ ⒸHCI What is the theoretical yield of Cl₂? theoretical yield: If the yield of the reaction is 72.1%, what is the actual yield of chlorine? actual yield: Resources g Carrow_forward37R37S nL of OilooM ealetum chloride reacts complalely with aques lyer nitrate iuhat s the 143.32 al mol) O.100M eaet (१०७.९ Co९० ) mass of Aa C! precipitale? Caclalag)+2arrow_forwardhrome File Edit View History Bookmarks Profiles Window Help A 34% O Tab Mon O Ask Laftan Anlamaz - Episode x O st. John's University - My Ap x A ALEKS - Iffat Khan - Learn D YouTube G whats an iv - Google Search www-awn.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNsIkr7j8P3jH-lijkPWvZoZLqKt1FLIq7wcPWKzBYGfE9IMFjvvh3x_rz6naF9YTN80Bms6y067EgyeOUwHKJOrzx. O STOICHIOMETRY Using molarity to find solute moles and solution volume Calculate the volume in milliliters of a 0.731 mol/L sodium nitrate solution that contains 150. mmol of sodium nitrate (NaNo,). Round your answer to 3 significant digits. のarrow_forward
- The great French chemist Antoine Lavoisier discovered the Law of Conservation of Mass in part by doing a famous experiment in 1775. In this experiment Lavoisier found that mercury(II) oxide, when heated, decomposed into liquid mercury and an invisible and previously unknown substance: oxygen gas. 1. Write a balanced chemical equation, including physical state symbols, for the decomposition of solid mercury(II) oxide (HgO) into liquid mercury and gaseous dioxygen. 2HgO + heat 2Hg (1) + O2(g) 2. Suppose 40.0 mL of dioxygen gas are produced by this reaction, at a temperature of 110.0 °C and pressure of exactly 1 atm. Calculate the mass of mercury(II) oxide that must have reacted. Be sure your answer has the correct number of significant digits. X x10 A 18 Ar 8.arrow_forwardpackets of the anhydrous form of a hydrate are sometimes used to keep cellars from being damp. Is there a limit to how long a packet could be used?arrow_forwardA miner’s lamp burns acetylene (C2H2) that is produced from water dripping onto solid CaC2. The other product formed from the reaction is calcium hydroxide. What volume of water (d = 1.00 g/mL) is required to react with 12.5 g of calcium carbide (CaC2) in the lamp?arrow_forward
- 13. Nitric acid, HNO3, is a strong acid in aqueous solutions. It is sold commercially as a concentrated solution with a density of 2.14 kg.L-¹ and containing 58% mass of HNO3. We introduce 10.0 mL of this solution in a 250.00 mL volumetric flask which is then filled to the mark with distilled water. This solution is called S. S is then diluted 100 times to afford solution S'. a. What is the definition of a strong acid? | b. What is the concentration of solution S"? c. What is the pH of solution S'?arrow_forwardChlorine can be prepared in the laboratory by the reaction of manganese dioxide with hydrochloric acid, HCI(aq), as described by the chemical equation MnO, (s) + 4 HCI(aq) MnCl, (aq) + 2 H,0(1) + Cl, (g) How much MnO, (s) should be added to excess HCl(aq) to obtain 205 mL CI, (g) at 25 °C and 735 Torr? mass of MnO2: garrow_forwardOCHEMICAL REACTIONS Dilution A chemist must prepare 875. ml. of 335. M aqueous copper (11) fluoride (CuF) working solution. She'll do this by pouring out some 407. µμM aqueous copper (11) fluoride stock solution into a graduated cylinder and diluting it with distilled water. Calculate the volume in ml. of the copper (11) fluoride stock solution that the chemist should pour out. Round your answer to 3 significant digits. 0 04 0/5 Yarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY