
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:II
5:44 PM Fri Oct 8
AA
www-awn.aleks.com
G periodic table -.
Favorites - YouT...
TV fuboTV - Watch...
ALEKS - Caliyah...
G kno3 acid or ba...
G linear program...
G write formulas f...
C Solu
O CHEMICAL REACTIONS
Predicting precipitation
Caliyah -
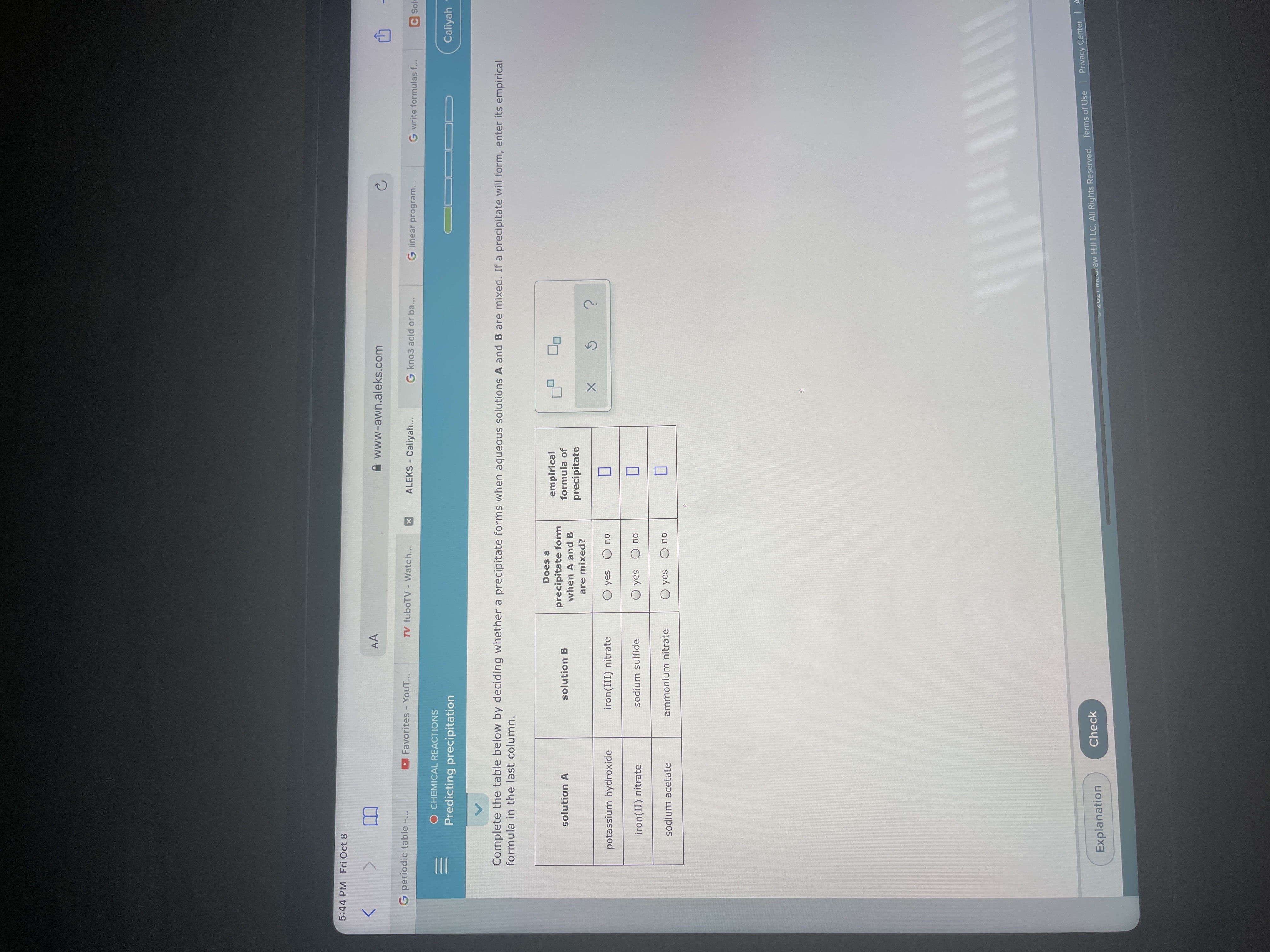
Complete the table below by deciding whether a precipitate forms when aqueous solutions A and B are mixed. If a precipitate will form, enter its empirical
formula in the last column.
Does a
precipitate form
when A and B
empirical
formula of
solution A
solution B
precipitate
are mixed?
potassium hydroxide
iron(III) nitrate
O yes
ou
iron(II) nitrate
sodium sulfide
ou O sək O
sodium acetate
ammonium nitrate
ou O sək O
Check
Explanation
22 COiaw Hill LLC. All Rights Reserved. Terms of Use I Privacy Center IA
Expert Solution
arrow_forward
Step 1
Given,
KOH + Fe(NO3)3  ?
?
Fe(NO3)2 + Na2S  ?
?
CH3COONa + NH4NO3  ?
?
Step by stepSolved in 3 steps with 5 images

Knowledge Booster
Similar questions
- Ff..181.arrow_forwardO CHEMICAL REACTIONS 1/5 Predicting precipitation Complete the table below by deciding whether a precipitate forms when aqueous solutions A and B are mixed. If a precipitate will form, enter its empirical formula in the last column. Does a precipitate form when A andB are mixed? empirical formula of precipitate solution A solution B potassium hydroxide zinc chloride O yes O no zinc acetate manganese(II) iodide O yes O no potassium acetate barium nitrate O yes O no IIIarrow_forwardcomplete the table below by deciding whether a precipitate forms when aqueous solutions A and B are mixed. If a precipitate will form, enter it's empirical formula in the last column.arrow_forward
- Complete the table below by deciding whether a precipitate forms when aqueous solutions A and B are mixed? If a precipitate will form, enter its empirical formula in the last column.arrow_forwardComplete the table below by deciding whether a precipitate forms when aqueous solutions A and B are mixed. If a precipitate will form, enter its empirical formula in the last column. Does a precipitate form when A and B empirical formula of precipitate solution A solution B are mixed? sodium hydroxide copper(II) bromide O yes O no sodium chloride ammonium bromide O yes O no ammonium sulfide zinc bromide O yes O noarrow_forwardComplete the table below by deciding whether a precipitate forms when aqueous solutions A and B are mixed. If a precipitate will form, enter its empirical formula in the last column. Does a precipitate form when A and B empirical formula of solution A solution B precipitate are mixed? zinc nitrate sodium hydroxide yes no iron(II) bromide zinc acetate yes no iron(II) chloride ammonium sulfide yes no Acces Check O 2021 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center Explanation MacBook Proarrow_forward
- Complete the table below by deciding whether a precipitate forms when aqueous solutions A and B are mixed. If a precipitate will form, enter its empirical formula in the last column. Does a empirical formula of precipitate form when A and B are mixed? solution A solution B precipitate manganese(II) bromide zinc nitrate O yes O no sodium sulfide O yes copper(II) sulfate no sodium hydroxide zinc nitrate O yes O noarrow_forward14. A student wants to determine the concentration of a AgNO3 solution. 200.0 mL of the AgNO3 solution is reacted with an aqueous NazSO4 solution until no more precipitate forms. A total of 5.4 grams of precipitate formed. What is the molarity of the AgNO3 solution? 2AGNO3(aq) + NazSO4(aq) 2NANO3(aq) + Ag:SO:(s) A) 0.085 M B) 0.17 M 1 C) 0.35 M D) 0.70 M E) 1.3 Marrow_forwardComplete the table below by deciding whether a precipitate forms when aqueous solutions A and B are mixed. If a precipitate will form, enter its empirical formula in the last column. solution A solution B Does a precipitate form when A and B are mixed? empirical formula of precipitate silver nitrate potassium iodide sodium chloride ammonium bromide potassium hydroxide copper(II) bromidearrow_forward
- Complete the table below by deciding whether a precipitate forms when aqueous solutions A and B are mixed. If a precipitate will form, enter its empirical formula in the last column. potassium acetate and ammonium bromide zinc sulfate and sodium sulfide potassium chloride and silver nitratearrow_forwardComplete the table below by deciding whether a precipitate forms when aqueous solutions A and B are mixed. If a precipitate will form, enter its empirical formula in the last column. Does a empirical formula of precipitate form when A andB are mixed? solution A solution B precipitate potassium acetate barium nitrate O yes O no iron(II) nitrate potassium sulfide O yes O no zinc nitrate sodium hydroxide O yes O noarrow_forwardpredict if one or more precipitates will form when aqueous solutions of chromium(II) chloride (CrCl2) and silver(I) nitrate (AgNO3) are mixed.Write the formula of any precipitate that could form in one of the boxes. If a box is not needed, leave it blank. If no precipitate is predicted, leave both boxes blank. predict if one or more precipitates will form when aqueous solutions of cobalt(II) acetate (Co(CH3COO)2) and zinc sulfate (ZnSO4) are mixed.Write the formula of any precipitate that could form in one of the boxes. If a box is not needed, leave it blank. If no precipitate is predicted, leave both boxes blank.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY