
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Predict the product where cyclohexadiene does a Diels-Alder with itself.
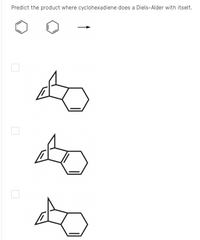
Transcribed Image Text:Predict the product where cyclohexadiene does a Diels-Alder with itself.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- True or False: Acetylene is a naturally occurring conjugated diene True or False: The Diels-Alder reaction has the stereochemistry of the dienophile is retained in the product. True or False: When looking at kinetic vs. thermodynamic products the kinetic product predominates at low temperature. True or False: the mechanism of the Diels-Alder reaction is three π bonds break; one σ bond and two π bonds form.arrow_forward7. At room temperature cyclopentadiene reacts with itself to form dicyclopentadiene in a Diels-Alder reaction. a) draw the self reaction of cyclopentadiene 20°C. b) When dicyclopentadiene is heated to boiling (170°C), the retor-Diels-Alder reaction occurs producing 2 moles of cyclopentadiene. Explain this observation in terms of free energy, enthalpy and entropy. c) If the AHxn = -75 kJ/mol and the ASpxn = -226 J/mol K, What temperature would be required for the reaction to be at equilibrium (Keq = 1).arrow_forwardDiels–Alder reaction of a monosubstituted diene (such as CH2=CH–CH=CHOCH3) with a monosubstituted dienophile (such as CH2=CHCHO)gives a mixture of products, but the 1,2-disubstituted product oftenpredominates. Draw the resonance hybrid for each reactant, and use thecharge distribution of the hybrids to explain why the 1,2-disubstitutedproduct is the major product.arrow_forward
- Following is an example of a type of reaction known as a Diels-Alder reaction 1,3-Pentadiene Ethylene 3-Methylcyclohexene (a racemic mixture) The Diels-Alder reaction between a diene and an alkene is quite remarkable in that it is one of the few ways that chemists have to form two new carbon-carbon bonds in a single reaction. Given what you know about the relative strengths of carbon-carbon sigma and pi bonds, would you predict the Diels-Alder reaction to be exothermic or endothermic? Explain your reasoning.arrow_forwardPlease show all arrow pushingj mechaaisais. Thank you!arrow_forwardMechanism The Diels-Alder reaction is part of a class of reactions known as a cycloaddition reaction. This reaction is specifically a [4+2] cycloaddition which is a concerted (one-step) process in which two new carbon - carbon sigma bonds are formed from two pi bonds. For the first Diels - Alder step of the mechanism fill in the arrows needed for the transformation. The rest of the mechanism is drawn for you. OH Show mechanism arrows for this step! 4 + 2 cycloaddition OH H D- & H+ transfer OH Nuc acyl substitution H L.G.arrow_forward
- Compounds P and Q can undergo a Diels-Alder reaction to form two regioisomeric products R and S as shown in Figure 5. OMe O C8H12O2 R C8H12O2 S Figure 5 Draw the chemical structures of regioisomeric compounds R and S. Using possible resonance contributors of P and Q predict which of the two regioisomers will be favoured in the reaction. Using curly arrows, draw the mechanism for the reaction of P and Q to form the dominant regioisomer you have predicted in your answer to part (ii) above.arrow_forwardA Diels-Alder reaction that shows regioselectivity, but not stereoselectivityarrow_forwardImagine that you used isoprene as diene – in that case you don’t have to worry about assigning endo vs exo. Draw the "endo" and "exo" products of the Diels-Alder reaction between isoprene and maleic anhydride, and explain why the distinction is irrelevant here.arrow_forward
- Is the following true or false? To catalyse a Diels-Alder reaction a common approach involves the use of a Lewis acid capable of lowering the LUMO of the dienophile.arrow_forwardBased on the given information, determine the products of the Diels-Alder reaction.arrow_forward[Review Topics] [References] Draw structural formulas for the diene and dienophile that combine in a Diels-Alder reaction to form the product shown. Diene + Dienophile CH3O CH₂ I wwwwwwwarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
