
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
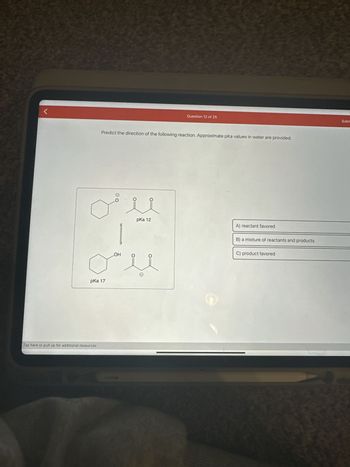
Transcribed Image Text:<
یدم
Predict the direction of the following reaction. Approximate pka values in water are provided.
pKa 17
Tap here or pull up for additional resources
OH
pKa 12
Question 12 of 25
ند
A) reactant favored
B) a mixture of reactants and products
C) product favored
Subm
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the parent of the following reaction (which element is X): 4X→ _e + Sr 87 -1 Rb-87 O Y-86 O Rb-86 O Y-87arrow_forwardSelect the atom that acts as the acid in the third step of the reaction. H × Selected Answer - Incorrect Η Η Η· H Η Ηarrow_forwardy courses / S22 CHEM 161_01_04 / 27 March - 2 April/ Quiz 9 Time left 0:53:13 Calculate the mass of oxygen that will be prepared through the decomposition of 50 kg of potassium chlorate ( KCIO3 , Molar mass= 122.55 g/mol) 2 KCIO3 →2 KCI + 3 O2 Select one: O a. 612 kg O b. 12.25 kg O c. 9.8 kg O d. 19.6 kg Next pagearrow_forward
- 3.25 mL of Biomarker A (4.21 x 10-7 mol L-1) was brought up to volume with deionized water in a 50 mL volumetric flask. What is the final concentration after this dilution (in mol/ L)arrow_forwardpKal pK₂2 Phosphoric acid, H, PO, (aq), is a triprotic acid, meaning that one molecule of the acid has three acidic protons. Estimate the pH and the concentrations of all species in a 0.300 M phosphoric acid solution. 2.16 7.21 [H, PO₁] = M [H*] = [H₂PO¡] = M [OH-] = [HPO ] = M pH = [PO] = M PK₂3 12.32 M Marrow_forwardGiven the reaction of 2 Ti (s) + 4 Cl2 (g) → 2 TiCl, (1) ΔΗ- 1608.4 J what would the AH for Ti (s) + 2 Cl, (g) TiCl, (1) S. -3216.8 J 804.2 J -804.2 J O-1608.4 J 5:18 PMarrow_forward
- Illustrate the process of determining the direction of equilibrium using pKa ?arrow_forwardThe donated proton from acids reacts strongly with non-binding electron pairs of water 000 0 hydroxide alcohol coke to form hydrated hydrogenarrow_forwardWhich statement about the pKa value is false? The pKa value is calculated from the equilibrium constant for the dissociation of the acid. The pKa value is a measure of the strength of an acid. The pKa value of a given acid gives an indication of the strength of the conjugate base of that acid. A larger pKa value indicates a stronger acid and a weaker associated conjugate base. All of these statements are correct.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY