
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
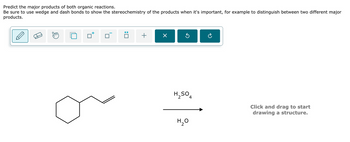
Transcribed Image Text:Predict the major products of both organic reactions.
Be sure to use wedge and dash bonds to show the stereochemistry of the products when it's important, for example to distinguish between two different major
products.
+
Х
H₂SO4
H₂O
Click and drag to start
drawing a structure.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Predict the major products of both organic reactions. Be sure to use wedge and dash bonds to show the stereochemistry of the products when it's important, for example to distinguish between two different major products. 田 گی گی B 田 : + : + G но Click and drag to start drawing a structure. ☑ а 心 1. BH3. THF 2.H₂O₂. NaOH Click and drag to start drawing a structure.arrow_forward+ 5 Predict the major products of both organic reactions. Be sure to use wedge and dash bonds to show the stereochemistry of the products when it's important, for example to distinguish between two different major products. : ☐ 円 H₂O Click and drag to start drawing a structure. H2SO4 ☐ : ☐ + х G 0 H2SO4, H₂O HgSO 4 Click and drag to start drawing a structure.arrow_forwardPredict the major products of both organic reactions. Be sure to use wedge and dash bonds to show the stereochemistry of the products when it's important, for example to distinguish between two different major products. ☐ 口 + х 5 H2SO4 H₂O Click and drag to start drawing a structure. 00 Earrow_forward
- Predict the major products of this organic reaction. Be sure you use wedge and dash bonds when necessary, for example to distinguish between major products with different stereochemistry. : ☐ + Х RCO₂H 3 Click and drag to start drawing a structure.arrow_forwardA certain hydrocarbon had the molecular formula C18H30 and contained two triple bonds. Ozonolysis gave CH₂(CH₂)CO₂H and HO₂CCH₂CH₂CO₂H as the only products. Draw a reasonable structure for this hydrocarbon. Click and drag to start drawing a structure.arrow_forwardN,N-diethyl-m-toluamide (DEET) is the active ingredient in many insect repellent preparations. Following is one of the steps in its synthesis. In the box below draw the structure of the product of this reaction. H3C MgBr 1. CO2 2. H3O+ product • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • Do not include lone pairs in your answer. They will not be considered in the grading. • Draw the Grignard reagent as a covalent magnesium bromide. 90-87 0 + 11 ? n [arrow_forward
- Draw a structural formula for the substitution product of the reaction shown below. H/ ● (CH3)3C |YY Br ΔΟ H Na • Use the wedge/hash bond tools to indicate stereochemistry where it exists. • If more than one stereoisomer of product is formed, draw both. Separate multiple products using the + sign from the drop-down menu. • Products that are initially formed as ions should be drawn in their neutral forms. SH acetone [Farrow_forwardDraw a structural formula for the substitution product of the reaction shown below. ● H3C HH Br + O • Use the wedge/hash bond tools to indicate stereochemistry where it exists. • If more than one stereoisomer of product is formed, draw both. Separate multiple products using the + sign from the drop-down menu. • Products that are initially formed as ions should be drawn in their neutral forms. ▼ In [F OCH 3 -85 ? Na CN CH3CH₂OH ChemDoodleⓇ >arrow_forwardAlcohols can be converted to alkyl bromides using PBr3, which causes a complete inversion of stereochemistry. OH 10 PBr 3 Draw the stepwise mechanism for bromination of an alcohol. Be sure to include non-zero formal charges and lone pairs as necessary. : OH Br of 0 Br. Br Br Add/Remove step X Click and drag to st= drawing a structurarrow_forward
- Draw structural formulas for the major organic product of the reagents shown. ÇI H2SO4. HNO3 HO, You do not have to consider stereochemistry. Apply formal charges to any nitro groups. If there is more than one major product possible, draw all of them. Separate multiple products using the + sign from the drop-down menu.arrow_forwardDraw a structural formula for the major product of the reaction shown.arrow_forward1. The acid-catalyzed elimination of an alcohol is an equilibrium reaction and requires special reac- tion conditions to favour the formation of the alkene product. What chemical principle controls the amount of alkene product that is produced? Provide two laboratory techniques that can be used to obtain good alkene yields in alcohol elimination reactions.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning