
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
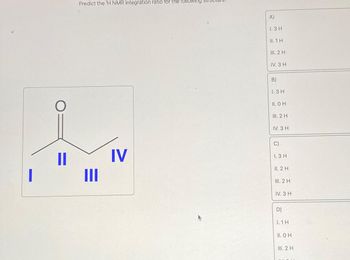
Transcribed Image Text:I
O
11
Predict the ¹H NMR integration ratio for the following
III
IV
A)
1.3 H
II. 1 H
III. 2 H
IV. 3 H
B)
1.3 H
II. O H
III. 2 H
IV. 3 H
C)
1.3 H
II. 2 H
III. 2 H
IV. 3 H
D)
1.1H
II. OH
III. 2 H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- predict the shift for the carbon indicated by the arrows please. USE NMR SPECTRUM TABLE please.arrow_forwardwhich structure is consistent with the hNMR spectrumarrow_forwardching and le X A PLQ-PHY2054L.docx - Google X engagenow.com/ilrn/takeAssignment/takeCovalentActivity.do?locator=Dassignment-take ... Translate Open Vellum Course Home [Review Topics] [References] Splitting of a signal in a proton NMR spectrum tells us the number of chemically non-equivalent hydrogens in the immediate vicinity of the hydrogen giving the signal. Predict the number of lines exhibited by hydrogens at the labeled positions in a first-order NMR spectrum. (Make the approximation that all coupling constants are equal.) 1) b a The number of lines exhibited by hydrogen(s) a is The number of lines exhibited by hydrogen(s) b is The number of lines exhibited by hydrogen(s) c is 2) b NH, The number of lines exhibited by hydrogen(s) a is The number of lines exhibited by hydrogen(s) b is The number of lines exhibited by hydrogen(s) c is Darrow_forward
- 22. What apparent splitting pattern would not be found in the 'H NMR spectrum or the following molecule? Singlet Doublet Triplet Quartet Quintet a. d. b. e. C. REF: 16.10 OBJ: Determine a simple splitting pattern using the N+1 rule. 23. Which molecule has a triplet of doublets in its 'H NMR spectrum? II Al a. AI b. e. II c. III REF:16.12 OBJ: Determine the complex splitting patterns that result when nonequivalent protons split a signal.arrow_forward9. Determine the structure of a compound with the following spectra and a formula of CSH12O2. Show your reasoning 3.5 3.0 2,0 1.5 1.0 PPM 0.5 2.5 Integral Values 15.5 80 Cwin 13NMR 160 120 100 20 FPM 100 68 2500 2000 1500 1000 WAVENURDERS Copyrisht 1992 600.0 caplary #is between sait pletes Spectra from A spectrum of Spectra, CD version, by Richard A Tomasi scat C21 18CT TADE 1745 1962 *TRANSKITTANCEarrow_forwardGiven the information about the following acids i) HW p Ka =2 ii) HX p Ka =6 iii) HY p Ka =17 iv) HZ p Ka =20 Which of these acids reacts almost completely with water to form hydroxide ion none all of them HZ HY and HZarrow_forward
- OWLV2 | Online teaching and le X D https://cvg.cengagenow.com/ilrm/takeAssignment/takeCovalentActivity.do?locator=Dassignment-take rome YouTube OTranslate Open Vellum Course Home [References] The 'H-NMR of a compound with molecular formula C4H,Br consists of one signal: 1.8 (singlet, integrating to 9 Hydrogens). Propose a structural formula for this compound consistent with this information. • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. C ору aste C.arrow_forwardAnalyze the HNMR data attached and state all take aways possible (stating all protons, chemical shift, multiplicity, integration, and coupling constants if necessary)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY