
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
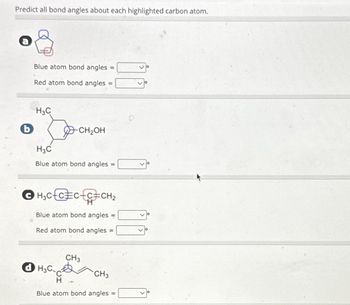
Transcribed Image Text:Predict all bond angles about each highlighted carbon atom.
b
Blue atom bond angles =
Red atom bond angles =
H3C
-CH₂OH
H3C
Blue atom bond angles
ⒸH₂CC=CC=CH₂
Blue atom bond angles =
Red atom bond angles =
dH3C.
CH3
CH3
Blue atom bond angles =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 8 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- omplete the Lewis diagram before answering the following questions. How many sigma bonds are there in a molecule of allylcyanide? How many valence electrons are there in a molecule of allylcyanide? How many pi bonds are there in a molecule of allylcyanide?arrow_forwardCan you help me with the bottom of the table. The last 3 ones (H2O, NH3, NH4+)arrow_forwardPredict the relative bond angles in BF3BF3 and SO2SO2. BF3BF3 bond angles >> SO2SO2 bond angle SO2SO2 bond angles >> BF3BF3 bond angle BF3BF3 bond angles == SO2SO2 bond angle Relative bond angles cannot be predicted.arrow_forward
- Subject--chemistryarrow_forwardIdentify the molecular geometry around the carbon atom highlighted red in the given organic molecule. CH3 H H 4-0-0 H Select one: H H Bent O Trigonal pyramidal Tetrahedral O Trigonal planararrow_forward4. Draw the complete Lewis structures showing all valence electrons, all atoms, all charges, all resonance forms for the molecules or species where appropriate. Show the best structure based on formal charge considerations. Supply the information requested. Water Ethanol (CH3CH₂OH) H-O-H Bond Angle Electronic Geometry of O atom Formate Ion (HCO₂¹) Carbon dioxide Electronic Geometry of C atom Hybridization of the Carrow_forward
- Please help me with number 5. I need the lewis structure, electron geometry, molecular geometry, and lewis structure indicating molecular geometry.arrow_forwardCompound # of bonds # of lone pairs H20 Single Double Triple # of electron clouds Electron-group Molecular shape Bond Anglearrow_forwardassign the electronic geometry and molecular geometry for the C bound to one C and three hydrogen in the following molecule EG: trigonal planar, MG: bent EG: tetrahedral, MG: tetrahedral EG: trigonal planar, MG: trigonal planar EG: tetrahedral, MG: trigonal planar EG: tetrahedral, MG: trigonal pyramid EG: tetrahedral, MG: bentarrow_forward
- 6. Use VSEPR to predict the geometry and bond angle for each bolded atom in the following structures. HNO₂ H₂C=CHCI CH3CCHarrow_forwardQuestion attachedarrow_forwardWhich one of the following covalent compound structures is characterized by the SMALLEST bond angles between pendant hydrogen atoms? H₂O dihydrogen monoxide H-O: H H₂O CH4 NH3 CH₂ carbon tetrahydride H H-C-H H NH₂ nitrogen trihydride H-N-H -I Harrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY