
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
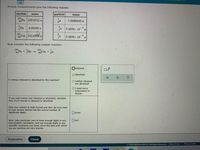
Transcribed Image Text:Precise measurements give the following masses:
nuclide
particle
mass
mass
239.
94
4 Pu 239.0522 u
on
1.0086649 u
He 4.00260 u
je 5.4858 x 10
*
u
242
96 Cm 242.058u
-4
5.4858 x 10u
Now consider the following nuclear reaction:
239
He 06 Cm + on
242
94 Pu +
Oreleased
O absorbed
Is energy released or absorbed by this reaction?
O neither released
nor absorbed
OI need more
information to
decide.
If you said energy was released or absorbed, calculate
how much energy is released or absorbed.
Give your answer in both kJ/mol and MeV. Be sure each
of your answer entries has the correct number of
significant digits.
OJmol
MeV
Note: take particular care to keep enough digits in any
intermediate calculation, and use enough digits in any
scientific constants you need, since the data with which
you are working are very precise.
Explanation
Check
O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The table below lists information about the radioactive decay of three nuclides. Fill in the missing information. nuclide 147 62 8 3 Sm Li decay mode alpha emission positron emission (choose one) ✪ daughter nuclide 0 11 B 8 4 Be Śarrow_forwardUnderstanding the common modes of radioactive decay The table below lists information about the radioactive decay of three nuclides. Fill in the missing information. nuclide decay mode 3 H 1 (choose one) 238 U 92 alpha emission daughter nuclide 3 He 2 ப positron emission 38 18 Ararrow_forwardUnderstanding the common modes of radioactive decay The table below lists information about the radioactive decay of three nuclides. Fill in the missing information. nuclide decay mode 180 W 74 ✓ (choose one) alpha emission 14 6 alpha absorption beta emission ☐ beta absorption positron emission negatron absorption electron capture proton capture daughter nuclide 176 Hf 72 88 ☐ 38 St ☑arrow_forward
- Provide the particle that is emitted by the following nuclear reaction. (Just type Alpha, Beta, Gamma, or Positron) 200 80 'Hg 196 Pt + 78 For Blank 1 Alpha * Pt + 222 | 86 Rn 222 85 Positronarrow_forwardMacmillan l 30: F2 W S A 210.2 ng sample of an unknown radioactive substance was placed in storage and its mass measured periodically. After 47 days the amount of radioactive substance had decreased to 26.28 ng. How many half-lives of the unknown radioactive substance have occurred? number of half-lives: #3 H 80 F3 mmand E D A GA $ X C 4 OOD 886 F4 R F % от ото 5 V F5 T G ^ 6 B F6 Y & 7 H 8 F7 U N 00 * 8 DII FB J L - ( 9 M K DD F9 O . V - ... I 4 F11 { [ option + = ? 11 1 F12 } ] half-lives delete return starrow_forwardCan you please assist me this problem, the answer that I just provided is wrong.arrow_forward
- The table below lists information about the radioactive decay of three nuclides. Fill in the missing information. nuclide 238 92 22 11 U Na 0 decay mode (choose one) positron emission alpha emission daughter nuclide 234 90 143 60 Th Nd X Śarrow_forwardQ6arrow_forwardneed help with this chemistry question, thanksarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY