
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
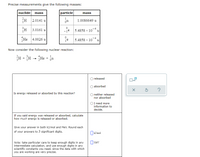
Transcribed Image Text:Precise measurements give the following masses:
nuclide mass
particle
mass
2.0141 u
1.0086649 u
3.0161 u
-je 5.4858 x 10¯* u
He
4.0026 u
5.4858 x 10
u
Now consider the following nuclear reaction:
H + H - He + ja
1
on
released
absorbed
Is energy released or absorbed by this reaction?
O neither released
nor absorbed
I need more
information to
decide.
If you said energy was released or absorbed, calculate
how much energy is released or absorbed.
Give your answer in both kJ/mol and MeV. Round each
of your answers to 3 significant digits.
OkJ/mol
O Mev
Note: take particular care to keep enough digits in any
intermediate calculation, and use enough digits in any
scientific constants you need, since the data with which
you are working are very precise.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Identify the unknown particle X in the following equation for fission. (Enter your answer in the form A X Z .) 23692U → X + 9436Kr + 3 10narrow_forwardq eq creq 2req 2req 2req 3 # E D Fill in the nuclide symbol for the missing particle in the following nuclear equation. He+310T1 Q C Submit Answer $4 R F V Retry Entire Group 9 more group attempts remaining % 5 [Review Topics] [References] Use the References to access important values if needed for this question. T Cengage Learning Cengage Technical Support G ^ 6 R MacBook Pro Y H & 7 N U * 00 8 J LE 1 M ( 9 K A O V ) C ** L P A Previous Email Instructor > + 11 { = [ Next> Save and E 11 ? #arrow_forwardMacmillan l 30: F2 W S A 210.2 ng sample of an unknown radioactive substance was placed in storage and its mass measured periodically. After 47 days the amount of radioactive substance had decreased to 26.28 ng. How many half-lives of the unknown radioactive substance have occurred? number of half-lives: #3 H 80 F3 mmand E D A GA $ X C 4 OOD 886 F4 R F % от ото 5 V F5 T G ^ 6 B F6 Y & 7 H 8 F7 U N 00 * 8 DII FB J L - ( 9 M K DD F9 O . V - ... I 4 F11 { [ option + = ? 11 1 F12 } ] half-lives delete return starrow_forward
- Complete these nuclear reactions with the particle that is emitted. {H – H+ Answer Bank 29 Сu Cu – Zn + 30- P n α 210 Po 206 Pb + 84 82arrow_forwardI need help balancing these nuclear equations. Thank youarrow_forwardOn absorption of a single neutron, an isotope of uranium U-235 can undergo fission into barium Ba-141 and krypton Kr-92, some neutrons, and 173 MeV of energy. The masses of these nucleiare, mU-235 = 235.044 amu, mBa-141 = 140.914 amu, and mKr-92 = 91.926 amu. Find the number of neutrons given off in this nuclear reaction.arrow_forward
- need help with this chemistry question, thanksarrow_forwardX * Cengage Digital Learning Fill in the missing symbol in X www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-Ivd38R-w7xPuAcyA O NUCLEAR CHEMISTRY Balancing a nuclear chemical equation Fill in the missing symbol in this nuclear chemical equation. 1. H' + 0 H + o - ƏH + 0 2. 8. Check Explanationarrow_forwardQ2arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY