
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
post-lab Question
Describe the 1H-NMR spectrum of p-bromoacetanilide (chemical shift, integration, multiplet). Discuss in 2-3 sentences typed.
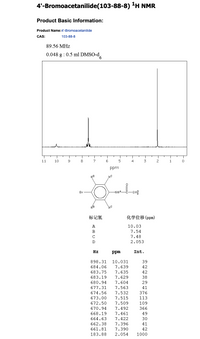
Transcribed Image Text:**4'-Bromoacetanilide (103-88-8) \(^{1}\text{H}\) NMR Analysis**
**Product Basic Information:**
- **Product Name:** 4'-Bromoacetanilide
- **CAS:** 103-88-8
**NMR Experimental Conditions:**
- **Frequency:** 89.56 MHz
- **Sample Preparation:** 0.048 g in 0.5 ml DMSO-d₆
**NMR Spectrum Overview:**
- The spectrum displays the chemical shifts in ppm along the x-axis, with intensity on the y-axis.
- Key peaks are observed, indicating different hydrogen environments in the molecule.
**Molecular Diagram:**
- The molecule features a benzene ring substituted with a bromo group and an acetamido group.
- The hydrogen atoms are labeled \( \text{H}^{\text{A}}, \text{H}^{\text{B}}, \text{H}^{\text{C}}, \text{H}^{\text{D}} \).
**Peak Assignments:**
- **\( \text{H}^{\text{A}} \):** 10.03 ppm
- **\( \text{H}^{\text{B}} \):** 7.54 ppm
- **\( \text{H}^{\text{C}} \):** 7.48 ppm
- **\( \text{H}^{\text{D}} \):** 2.053 ppm
**Detailed Peak Positions and Intensities:**
- 898.31 Hz (10.031 ppm), Intensity: 39
- 684.06 Hz (7.639 ppm), Intensity: 42
- 683.75 Hz (7.635 ppm), Intensity: 48
- 683.19 Hz (7.629 ppm), Intensity: 38
- 680.94 Hz (7.604 ppm), Intensity: 29
- 677.31 Hz (7.563 ppm), Intensity: 471
- 674.56 Hz (7.532 ppm), Intensity: 376
- 673.00 Hz (7.515 ppm), Intensity: 113
- 672.50 Hz (7.509 ppm), Intensity: 109
- 670.94 Hz (7.492 ppm), Intensity:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- From the spectra A-J and in the NMR Spectra tile, select the letter that corresponds to 1. methyl butanoate2. benzaldehyde3. 1-chlorobutane4. 1-chloro-2-methylpropane5. butan-2-one6. propan-2-ol7. propanalarrow_forwardAnalyze and label this NMR spectrum of butyl propanoatearrow_forwardIdentify the fragments of hexanoic acid in the mass spectrum.arrow_forward
- urgent :i am supposed to match the peaks on the compound to where it is on the nmr spectrscopy. i started matching but am not sure if i am right, please help finish and correct if wrongarrow_forwardPeaks in the NMR spectrum for the product ester: report the center of a multiplet as the chemical shift, the integration (relative intensity), and the multiplicity (singlet, doublet, triplet) for each unique peak set.arrow_forwardHow can I find the numbers of hydrogens per each peak on the NMR chart of the image?arrow_forward
- 2. READ THE DIRECTIONS TO EACH QUESTION CAREFULLY - not following the directions means you get the question wrong! 3. FORMAT YOUR ANSWERS AS DIRECTED - formatting your answers incorrect means you get the question wrong! 4. Below are the IR regions and NMR chemical shifts table for use with the spectroscopy problems: ¹H NMR 12 R ΟΞΟ 11 10 PIC OH R H 9 8 7 ppm 6 OH H C=C-H 5 NH H-C-X H-C-O -OH X = F, Cl, Br (i.e. electronegative atom) 3 -NH 2 H-C-N H-C-S 1 -CH3 -CH₂- C=C-H H-C-C=O -CH H-C-C=C HC-0 0 4000 small range Hrange of values broad peak DID =C-H N-H 3250- 3300 broad with spikes -3300 3500 -O-H broad-3300 -3400 -O-H CEN usually strong N-H CIO C H ₹ -C-H O 2730- 2820 3000- 2 peaks 3100 2850- 2960 O -C-O-H broad-3000 3000 H -CEN 2500 wavenumber, cm-1 2200 <-CICH B 2200 2000 CC H.C. N 1680 000000 H 1600- 1660 0 OR 1730 1710 1600 NR₂ 1650 1500 1000arrow_forward3- Show (draw) or print or bring the 1H-NMR spectra and 13C-NMR spectra for diethylETHER.arrow_forwardC7H12O4 I need help answering 2 3 4 5 For IHD please show step by step ir analysis, frequency, functional group ester, alkane etc. appearance like strong medium weak 5 I need to match the peaks with the proton structurearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY