
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Help

Transcribed Image Text:For this problem you must identify the missing intermediates in the mechanism for the reaction shown below, which converts the starting reactant to product in seven mechanistic steps via six mechanistic intermediates; th
structure of one of the six intermediates (number V) has been provided for you,
NaOH,
H20
Ph
H3C
Ph
Ph
CH
Ph
Ph
CH2
CH
reactant
product
Possible mechanistic intermediates (A - 1) are shown directly below - the five correct missing intermediates are contained within this group of structures.
Ph
HC
Ph
Ph
CH3
Ph
CH
CHa
Ph
CH
CH
Ph
Ph
Ph
CH3
Ph
CH2
CH
Ph
HO
Ph
Ph
CH2
CH
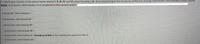
Transcribed Image Text:To signify your choices, in the spaces below labeled I, II, III, IV, and VI, place the letters (A - 1) corresponding to the structures of the five missing mechanistic intermediates shown above.
NOTE: mechanistic intermediates must be placed in the correct order!
mechanistic intermediatel-
mechanistic intermediate II =
mechanistic intermediate III =
mechanistic intermediate IV =
mechanistic intermediate V = already provided in the mechanistic sequence above
mechanistic intermediate VI =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- If the caffeine concentration in a particular brand of soda is 3.37 mg/oz, drinking how many cans of soda would be lethal? Assume that 10.0 g of caffeine is a lethal dose, and there are 12 oz in a can. cans of soda:arrow_forwardcan you help with 16, 17, and 18 as well please?arrow_forwardInjecting an arterial fluid which is hypertonic to the moisture level of the body will ___. add more water to the tissues remove water from the body's tissues bring salt and sugar molecules into the capillaries, they are removed in the venous drainage cause decomposition to progress fasterarrow_forward
- he solubility of oxygen in water is about 4.5 x 10-2 g/L. The water portion of an adult's total blood supply is about 5 liters. How many grams of oxygen could dissolve in 5 liters of water? A. 4.5 x 10-2 g/L B. 0.23 g/L C. 9.0 x 10-3 g/L D. 110 g/Larrow_forwardNonearrow_forwardNeed help with #2. (Info in #1 is needed to solve for #2)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY