
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
please show the mechanism
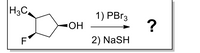
Transcribed Image Text:H3C,
1) PBr3
ОН
F
2) NaSH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Give a clear explanation handwritten answer...arrow_forwardFill in the missing information. ОН CH3 H+ НО Br- ОН m-CPBA ОН Br H2 Pd/C 1. NaBH4 2. H2O+ Іarrow_forwardWhich of the following are not allowed? State which rule is violated for any that are not allowed. (a) 1p3 (b) 2p8 (c) 3g11 (d) 4f2arrow_forward
- 3C. and 3Harrow_forward1. Fill in the boxes below with the correct reagent, reactant or product (5 (а) OH (b) 1. (CH),СHMgBr 2. H,0 ÓH (c) 1. L¡AIH, 2. НО (d) CH;OH H,SO4 (e) 1. 2 (CH3),CHMgBr, 2. H,0 ÓH (f) 1. LIAIH, 2. H,0 HO,arrow_forward4) H3C Br Br CH3 'CI + + HỈOH Н ОН Дон ОН SH КОН H2Oarrow_forward
- 6|Page NaH Br Downloaded by Tirelo Malapela (mologadi095@gmail.com)arrow_forwardE11 NB Q6 ... Unanswered If burning a substance in pure oxygen gas makes the flame glow brighter, predict what would happen if you put the burning substance in a container without any oxygen gas. Your answer Write your response here... K Submitarrow_forwardA chemist fills a reaction vessel with 3.61 atm nitrogen (N,) gas, 8.64 atm oxygen (0,) gas, and 6.55 atm nitrogen monoxide (NO) gas at a temperature of 25.0° C. Under these conditions, calculate the reaction free energy AG for the following chemical reaction: N,(g) + O,(g) = 2NO (g) Use the thermodynamic information in the ALEKS Data tab. Round your answer to the nearest kilojoule. do Data HNO3 (1) -174.1 HNO3 (g) -133.9 Oxygen OH (aq) H20 (1) -285.8 H20 (g) -241.8 02 (9) H202 (1) -187.8 H202 (9) -136.3 Phosphorus Explanation Check P4 (s) 2021 McCarrow_forward
- What is the value of Ered when log3Ag+4 = 0?arrow_forwardH3C CI NABH, CH3 AICI, c)arrow_forwardIn your own words, describe why metals are such good conductors of electricity. Be sure to include the definition of metallic bond in your answer. Answer in 2 to 3 complete sentences. I !!! H Normal A Enter your answer here BIUS √x 20 Txarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY