
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
For each of the four reaction mechanisms (SN1, SN2, E1, E2) pick one of each to combine such that they are most likely to undergo reaction by that particular mechanism. You may also make a comment about temperature. Do NOT use anything more than once. You want to pick you combinations so that the indicated mechanism is most likely to occur-there will not be any “no reactions”. Where the mechanism is indicated draw the full arrow pushing mechanism and products of each reaction. If the indicated mechanism will form more than one isomer you should draw structures of all isomers which may be formed by the indicated mechanism but you do not need to draw the full mechanism for each. Pick any one to do the mechanism. You do NOT need to draw minor products formed yet. On the reactant molecules shown adding R and S to each chirality center.
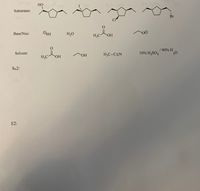
Transcribed Image Text:НО
10% SO
I ON
Substrates:
Br
CI
Base/Nuc:
OSH
H,O
H;C
ОН
/ 90% H
Solvent:
H;C-C=N
10% H2SO4
H,C-
ОН
ОН
SN2:
E2:
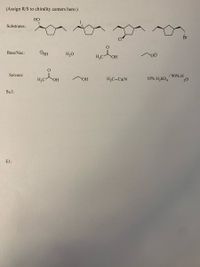
Transcribed Image Text:(Assign R/S to chirality centers here:)
НО
Substrates:
Br
Base/Nuc:
OSH
H,O
HO
Solvent:
H;C-C=N
10% H2SO4
/ 90% H
20
H;C
ОН
ОН
SNl:
El:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the following statement in reference to SN1, SN2, E1, and E2 reactions of haloalkanes. To which mechanism(s), if any, does the statement apply? Is first order in haloalkane and first order in nucleophilearrow_forwardAfter running various experiments, you determine that the mechanism for the following reaction is bimolecular. Br. + Using this information, draw the correct mechanism in the space below. Br + Br Add/Remove step X G Click and drag to start drawing a structure.arrow_forwardArrows are incorrect. Can you please explain the proper curved arrow movement for this mechanism?arrow_forward
- Draw the mechanism for the following reactions. i. [PtCla]? + NH3 → [PtCl3(NH3)] +Cl¯ (Association mechanism) ii. [Co(NH3)5Cl]2+ + H2O → [Co(NH3)5H2O]3+ + Cl (Dissociation mechanism)arrow_forwarddraw the tetrahedral intermediate as it is ormed in the following reaction. H₂C O NH₂ HCI/H₂O reflux You do not have to consider stereochemistry. Include all valence lone pairs in your answer. Do not include counter-ions, e.g., Na+, I, in your answe In cases where there is more than one answer, just drawarrow_forwardReaction Sequence and Mechanisms, thank you for your help.arrow_forward
- Nonearrow_forwardDid the following overall reaction occur by an SN2, Sn1, E2, or E1 mechanism? How do you know? Draw the complete, detailed mechanism to account for the formation of both products. H3C, H3C. H3C. + Brarrow_forwardPlease answer with All the required steps needed to solve itarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY