
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Please read the full question and answer all parts for the NMR.
Also, please complete the table NMR spectrum of Benzaldehyde and Pyridine. Please list the peak, chemical shift, multiplicity, and H based on the image of the NMR. Both molecules' spectra are represented in the images. Thank you.
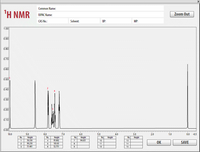
Transcribed Image Text:Common Name:
Η ΝMR
IUPAC Name:
Zoom Out
CAS No.:
Solvent:
ВР:
MP:
1.00
0.90
0.80
0.70
0.60
0.50
0.40
0.30
0.20
0.10
0.00
10.0
9.0
8.0
7.0
6.0
5.0
4.0
3.0
2.0
1.0
0.0
-0.5
No.
Height
50.330
No.
Height
95.802
No.
Height
94.635
No.
Height
No.
Height
4
7.
10
13
OK
SAVE
90.210
103.83
11
14
3
51.481
52.751
12
15
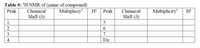
Transcribed Image Text:Table #: 'H NMR of (name of compound)
Multipliciy*
Peak
Chemical
Peak
Chemical
Multiplicity
H
Shift (8)
Shift (8)
1.
2.
3.
4.
Etc.
567E
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Masses of compounds used and observations NaCI CuSO4-5H20 Bright blue crystaline solids (NH4)2Fe(SO4)2 pale, Blue-green crystalline solid colorless Crystals Appearance of solid Mass of vial and 12.8364 12.7398 12.9445 compound (g) Mass of emptied vial (g) 11.6023 11.4888 11.9221 Mass of compound used (g) 1.2341. 1.2510. 1.0224 Volume of prepared solution = Type response here. Mass of sodium chloride used = Type response here.arrow_forwardgraph of melting temperature (°C) versus percent stearic acid by massarrow_forwardRichards and Willard determined the molar mass of lithium and collected the following data. Experiment Molar Mass, g/mol 1 6.9391 2 6.9407 3 6.9409 4 6.9399 5 6.9407 6 6.9391 7 6.9406 a. Find the mean molar mass determined by these workers. b. Find the median molar mass. c. Assuming that the currently accepted value for the molar mass of lithium is the true value (state your reference), calculate the absolute error and the percent relative error of the mean value determined by Richards and Willard.arrow_forward
- there is more than one correct answerarrow_forwardPart D: Food Calories Naked Juice Serving size 240 mL D1. Name of food product D2. Mass of food nutrients in one serving Fat Carbohydrate 33 g, Protein g, D3. Calculations for Calories (Cal) or kilocalories (kcal) per serving kcal (Cal) Carbohydrate Protein kcal (Cal) Fat kcal (Cal) D4. Total Calories (Cal or kcal) per serving kcal (Cal) D5. Calories (for one serving) listed on the label cal (Cal) D6. Percentage of total Calories from carbohydrate % (show calculations) D7. Percentage of total Calories from protein (show calculations) % D8. Percentage of total Calories from fat (show calculations) % Q 4. How does your calculated number of Calories in one serving compare to the Calories listed on the food product nutrition label? Comment on it.arrow_forward5. In the Dumas method for determining the molar mass of an unknown volatile liquid, a sample of a volatile liquid that boils below 100°C is vaporized in a boiling water bath $ (4 101 and the mass of the vapor required to fill the flask is determined. The following data was collected: Flask Volume Mass of Flask + Cap Mass of Flask + Condensed Liquid Mass of Condensed Liquid. Temperature of Water Bath [°C) Barometric Pressure (atm) Accessibility: Unavailable 15 a. What is the molar mass of the unknown? b. What will its temperature be when the pressure is 1.20 atm and the volume is 250.0 mL? EN 100.0 100.0 + 79.6 mL = 279.6 mL 143.85 g 144.95 g & 1.10 g 84.1 °C 0.985 atm CH 8 144 9 ho 11 Oarrow_forward
- 1a. Determine the volume of 1.000 g of water (Densitywater = 0.9999 g/mL). 1b. Determine the volume of 1.000 g of ice (Density ce = 0.9168 g/cm³). (1 mL = 1 cm³) %3D %3D 1c. From the volumes above, what can we conclude about 1.000 g of water when it freezes?arrow_forwardPlease don't provide handwriting solutionarrow_forwarddrc%3D0&gi=24758088ctgl=18dnb 06 What is the molarity of a solution prepared by dissolving 213.6 grams of Fe(OH)3 in enough water to produce a total volume of 1.5 L? Round your answer to 1 decimal place. Your Answer: Answer units D Add attachments to support your workarrow_forward
- Hi I just need to make a bar graph fr my data.arrow_forwardNewly produced beer must be checked to see if it meets predetermined standards. The percentage of alcohol is determined by distillation. A set of measurements is listed forone batch.Sample Volume of beer (L) Ethanol distilled (mL)1 8.00 3922 10.00 5003 9.00 4594 9.00 444 What is the average percentage by volume of ethanol for this batch?arrow_forwardMilliliters of isopropanol = mL Submit Part C How many liters of a 3.32 MK2SO4 solution are needed to provide 58.1 g of K2 SO4 (molar mass 174.01 g/mol)? Recall that M is equivalent to mol/L. Express your answer to th ree significant figures. View Available Hint(s) ? ΑΣΦ P Pearson Permissions Contact Us Copyright C2019 Pearson Education Inc. All rights reserved.| Terms of Use IPrivacy Policy F12 F11 II F8 F10 F9 F7 F6 F5 F4 F3 & A +II tarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning