
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please do C-NMR for this molecule

Transcribed Image Text:168.4561
138.6930
180 170 160 150 140
131.4157
120.8340
130 120
114.4085+
110
100
90
80
70
09
DMSO
50 40
30
23.9761
20
10
0
ppm
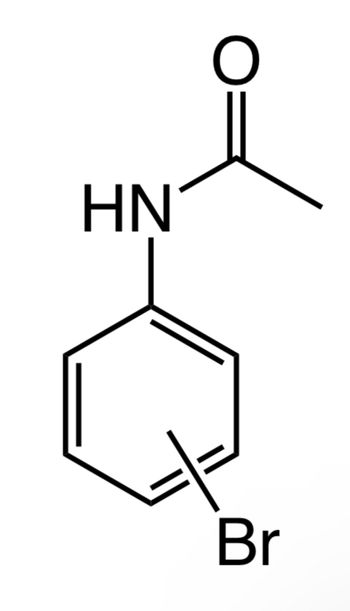
Transcribed Image Text:HN
0
Br
Expert Solution
arrow_forward
Step 1
Given that, a molecule is
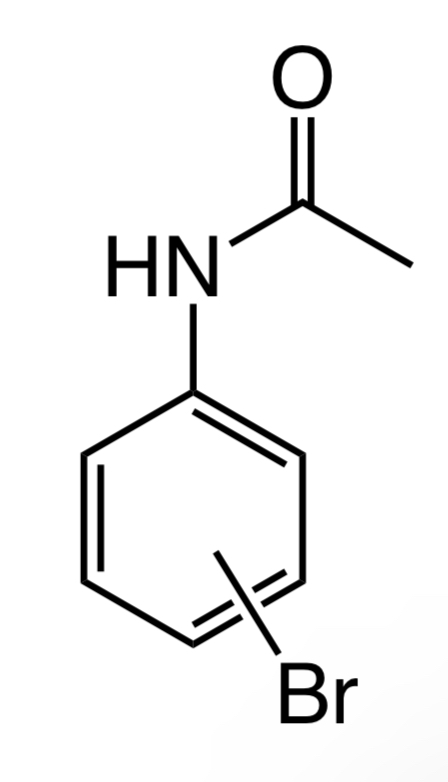
Also, the carbon NMR spectrum is shown below
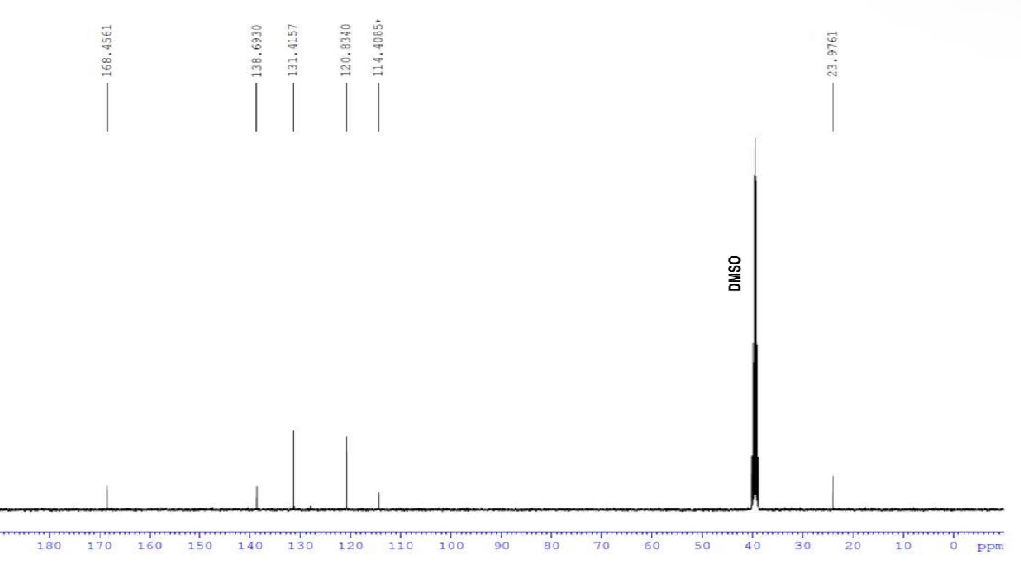
We have to assign carbon spectrum.
Step by stepSolved in 2 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- designate the following stretches in the IR of asprin : Csp3-H, Csp2-H, OH of carboxylic acid, C=O of acid, C=O of ester, benzene ring, C-Oarrow_forwardDetermine and draw in the missing R functional groupsarrow_forwardPredict and draw the major fragment structures and associated mass of each fragment for the following compounds.arrow_forward
- A 13C NMR spectrum is shown for a molecule with the molecular formula of C.HgO2. Draw the structure that best fits this data. 180 160 140 120 100. 80 60 40 20 "PPM Drawingarrow_forwardHow do I interpret, label, and determine which molecule this H NMR graph represents? The three molecule choices are methylene cyclohexane, 3-methylcyclohexene, or 1-methylcyclohexene.arrow_forward3- Show (draw) or print or bring the 1H-NMR spectra and 13C-NMR spectra for diethylETHER.arrow_forward
- 2. Circle the molecule below that matches the 'H NMR spectrum. 2.0 1.0 6.0 3.0 lle 3.0 2.5 2.0 1.5 1.0 0.5 0.0 Ō (ppm) re Br Brarrow_forwardEstimate the pKa for the proton highlighted. Type your answer... H CH₂arrow_forwardExplain why the pKa of compound A is lower than the pKa’s of both compounds B and C.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY