
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
help me
Transcribed Image Text:Please answer these questions on your Page 1
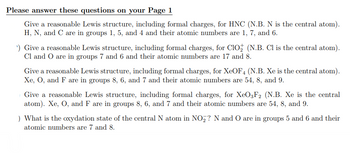
Give a reasonable Lewis structure, including formal charges, for HNC (N.B. N is the central atom).
H, N, and C are in groups 1, 5, and 4 and their atomic numbers are 1, 7, and 6.
¹) Give a reasonable Lewis structure, including formal charges, for ClO₂ (N.B. Cl is the central atom).
Cl and O are in groups 7 and 6 and their atomic numbers are 17 and 8.
Give a reasonable Lewis structure, including formal charges, for XeOF4 (N.B. Xe is the central atom).
Xe, O, and F are in groups 8, 6, and 7 and their atomic numbers are 54, 8, and 9.
Give a reasonable Lewis structure, including formal charges, for XeO3F2 (N.B. Xe is the central
atom). Xe, O, and F are in groups 8, 6, and 7 and their atomic numbers are 54, 8, and 9.
) What is the oxydation state of the central N atom in NO₂? N and O are in groups 5 and 6 and their
atomic numbers are 7 and 8.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The maximum safe level of a chemical in drinking water is determined to be 52 μg per kg of water. Convert this value to parts per million (ppm).arrow_forwardPart A: The graphic shows an organic molecule and highlights a functional group that is bonded to it. This functional group is an example of ______ group. Part B: select which Characteristic this functional group impacts on the molecule. A. Forms disulfide bonds B. Nonpolararrow_forward1. NABH4 H. 2. H-о FEB 20 MacBookarrow_forward
- Hello can I get help with this question soon please?!? I am very confused and do not know where to start. An explanation leading to the correct answer would be helpful. Thank you!arrow_forwardFor acetic acid (vinegar) explain the hazards effects of being exposed to this chemical either by contacting the skin, swallowing it, or contacting your eye. What should you do then as first aid and, describe how you should dispose off those chemicals.arrow_forwardI really don't know what I'm looking at so if I could get some help please.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY