
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
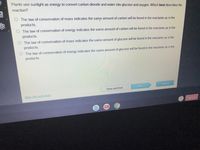
Transcribed Image Text:Plants use sunlight as energy to convert carbon dioxide and water into glucose and oxygen. Which best describes the
reaction?
The law of conservation of mass indicates the same amount of carbon will be found in the reactants as in the
products.
O The law of conservation of energy indicates the same amount of carbon will be found in the reactants as in the
products.
The law of conservation of mass indicates the same amount of glucose will be found in the reactants as in the
products.
O The law of conservation of energy indicates the same amount of glucose will be found in the reactants as in the
products.
Submit
Next
Save and Exit
Mark this and return
ano uis
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- One liter of gasoline at pump costs $1.2. One US gallon of E85 (85% ethanol + 15% gasoline by volume) at pump costs $3.3 (both in Canadian dollars). Traveling from location A to location B via a compact car consumes about 448 MJ of fuel. Which fuel (gasoline or E85) will cost less for this trip?arrow_forwardFor most automobiles, the number of miles per gallon decreases as highway speed increases. Fuel economy drops as speeds increase from 55 to 65 mph, then decreases further as speeds increase to 75 mph. Explain why this is the case.arrow_forwardA power plant has been proposed that would make use of the temperature gradient in the ocean. They system is to operate between 24.0°C (surface water temperature) and 4.95°C (water temperature at a depth of about 1 km). a. What is the maximum efficiency of such a system? Describe the process that would be used to get this maximum efficiency. b. If the useful power output of the plant is 80.0 MW, how much energy is absorbed per hour?arrow_forward
- Manufacturers of headache remedies routinely claim that their own brands are more potent pain relievers than the competing brands. Their way of making the comparison is to compare the number of molecules in the standard dosage. Tylenol uses 325 mg of acetaminophen (C8H9NO2) as the standard dose, while Advil uses 2.00 x 102 mg of ibuprofen (C13H1802). Find the number of molecules of pain reliever in the standard doses of (a) Tylenol and (b) Advil. (a) Number Units (b) Number Unitsarrow_forwardA 0.0545 kg chunk of an unknown metal that has been in boiling water for several minutes is quickly dropped into an insulating Styrofoam beaker that contains 0.462 kg of water at room temperature (20.0° C). After waiting for a few minutes, you observe that the water's temperature has reached a constant value of 22.0° C. Part A Assuming that the Styrofoam absorbs a negligibly small amount of heat and that no heat was lost to the surroundings, what is the specific heat of the metal? Express your answer in joules per kilogram-kelvin to three significant figures. ΑΣφ J/(kg · K) C = Submit Request Answer Part B Use the table below to identify the metal. Specific heat (c) Material J/(kg K) cal/(g K) Solids Lead 0.13 x 103 0.031 Mercury 0.14 × 103 0.033 Silver 0.23 × 103 0.056 Copper 0.39 × 10³ 0.093 Iron 0.47 x 103 0.112 Marble (CACO3) 0.88 × 10³ 0.21 Salt 0.88 × 103 0.21 Aluminum 0.91 × 103 0.217 Beryllium 1 97 x 103 0.471arrow_forwardPlease asaparrow_forward
- Electric vehicles increase speed by using an electric motor that draws energy from a battery. When the vehicle slows, the motor runs as a generator, recharging the battery. Explain why this means that an electric vehicle can be more efficient than a gasoline-fueled vehicle.arrow_forwardPlasma spraying is a process used to coat the surface of a material with a protective layer to prevent material degradation. In a plasma spray process, the protective coating in powder form is injected into a plasma jet. The powder is then heated until the droplets coalesce and are propelled over the surface of the material. Once deposited on the surface of the material, the molten droplets solidify and form a protective coating layer. Consider a plasma spray process using alumina (k = 30 W/m K, ρ = 3970 kg/m³ and cp = 800 J/kg K) powder that is injected into a plasma jet at T∞ = 15,000 ° C and h = 10,000 W/m²·K. Alumina powder is made up of spherical-shaped particles with a mean diameter of 60 μm and a melting point of 2300 °C.Solving, the amount of time it would take for the particles, with an initial temperature of 20 °C, to reach their melting point from the time they are injected into the plasma jet is 5,25x10 -4 s. The mathematical resolution is in the image. I only need the…arrow_forwardWhat is the ΔS° for the reaction? Given are the S°, in J K-1 mol-1, of each substance in parentheses. SiCl4 (g, 330.73) + 2 Mg (s, 32.68) → 2 MgCl2 (s, 89.62) + Si (s, 18.83) -198.02 J/K 198.02 J/K -254.96 J/K 254.96 J/Karrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON