
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
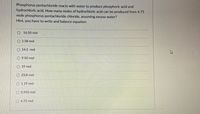
Transcribed Image Text:Phosphorus pentachloride reacts with water to produce phosphoric acid and
hydrochloric acid. How many moles of hydrochloric acid can be produced from 4.75
mole phosphorus pentachloride chloride, assuming excess water?
Hint, you have to write and balance equation
O 16.50 mol
1.58 mol
O 14.3 mol
O 9.50 mol
O 19 mol
O 23.8 mol
O 1.19 mol
O 0.950 mol
O 4.75 mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- 119 g of aluminum sulfide reacts with water to form aluminum hydroxide and hydrogen sulfide. Calculate the amount of aluminum hydroxide produced with excess water.arrow_forwardCombustion of hydrocarbons such as dodecane (C1,H26) produces carbon dioxide, a "greenhouse gas." Greenhouse gases in the Earth's atmosphere can trap the Sun's heat, raising the average temperature of the Earth. For this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide. 1. Write a balanced chemical equation, including physical state symbols, for the combustion of liquid dodecane into gaseous carbon dioxide and gaseous water. O-0 Ox10 2. Suppose 0.140 kg of dodecane are burned in air at a pressure of exactly 1 atm and a temperature of 12.0 °C. Calculate the volume of carbon dioxide gas that is produced. Round your answer to 3 significant digits.arrow_forwardWrite a balanced chemical equation based on the following description: butane gas and oxygen gas react to produce carbon dioxide gas and water vaporarrow_forward
- for the following reaction 28.3 grams of disphosphorus pentoxide are allowed to react with 15.0 grams of water. What is the maximum amount of phosphoric acid that can be formed?arrow_forwardDesigning Chemical Reactions Lab Activity Given the list of chemicals below, write a balanced chemical equation that satisfies the reaction described. Write a description of what signs of a chemical change you would expect to see if you were able to do the reaction and write the total and net ionic equations for each reaction. Metals Bases Compound Solutions. copper (II) sulfate Ferric nitrate Acids sulfuricic acid Magnesium sodium hydroxide Indicators phenolphthalein litmus paper Zinc Calcium potassium iodide potassium nitrate Copper Iron sodium carbonate magnesium nitrate iodine solution. Single Displacement Reactions: ● Reaction #1 - Pick one reaction that works using the metal activity series: Indication of a chemical change: Total lonic Equation: Net Ionic Equation: Reaction #2 - Pick one reaction that works with water: Indication of a chemical change: Total lonic Equation: Net Ionic Equation: Reaction #3 - Pick one reaction that does not work using the halogen series: Indication…arrow_forwardWrite a balanced metathesis equation for the reaction which takes place when the two solutions described below are combined and then determine the amount of solid product produced. 350.0 ml of 0.125 M solution of FeCl3 is combined with 475.0 ml of 0.600 M KOH solution. How many grams of solid iron (III) hydroxide will be produced by the reaction. Assume 100% yield. grams of iron (III) hydroxidearrow_forward
- 158 g of aluminum sulfide reacts with water to form aluminum hydroxide and hydrogen sulfide. Calculate the amount of aluminum hydroxide produced with excess water.arrow_forwardIf MgCl2·H2O is the limiting reagent with a mole amount of 0.00189 mole. What is the theoretical yield of Mg(OH)2 (58.319 g mol-1) precipitate which will be formed?arrow_forwardAmmonia (NH3) is a weak base. Melting point of ammonia is -78 Celsius degrees and boiling point is -33 Celsius degrees. Therefore, pure ammonia appears in gaseous form in room temperature. a) Preparation of ammonia Gaseous ammonia (NH3) can be prepared by a reaction where Hydrogen gas (H2) and Nitrogen gas (N2) reacts. Write a reaction equation to this reaction. Balance the reaction equation if needed. b) Behavior of gases Explain the diffusion of gases. c) Change of temperature The closed container filled with gaseous ammonia is moved from room temperature into a freezer. The temperature on freezer is -35 Celsius degrees. Explain shortly on your own words, what kind of changes does the cooling cause and why.arrow_forward
- To determine the mass of SbF₃ needed to produce 1.00 g of Freon-12 (CCl₂F₂), you can use the balanced chemical equation and the molar masses of the substances involved. The balanced chemical equation for the reaction is not provided, but assuming a hypothetical balanced equation:arrow_forwardWrite a balanced chemical equation for the combustion of hydrogen and oxygen to give water.arrow_forwardCombustion of hydrocarbons such as dodecane (C₁2H26) produces carbon dioxide, a "greenhouse gas." Greenhouse gases in the Earth's atmosphere can trap the Sun's heat, raising the average temperature of the Earth. For this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide. 1. Write a balanced chemical equation, including physical state symbols, for the combustion of liquid dodecane into gaseous carbon dioxide and gaseous water. 2. Suppose 0.290 kg of dodecane are burned in air at a pressure of exactly 1 atm and a temperature of 20.0 °C. Calculate the volume of carbon dioxide gas that is produced. Be sure your answer has the correct number of significant digits. L 010 X x10 Śarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY