
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
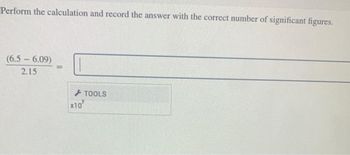
Transcribed Image Text:Perform the calculation and record the answer with the correct number of significant figures.
(6.5-6.09)
2.15
=
X10
TOOLS
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Conversion Factors in Calculations Report Sheet - Lab 2 B. Signlficant Figures in Calculations B.1 Perform the following multiplication and division calculations. Give a final answer with the correct number of significant figures: 4.5 x 0.28 0.1184 x 8.00 x 0.0345 (42.4)(15.6) 1.265 (35.56)(1.45) (4.8)(0.56) B.2 Perform the following addition and subtraction calculations. Give a final answer with the correct number of significant figures. Pre settin 2-Line entry of up Entry Line). 11 digits scrolf line. Press 2nd immediately to the The second line (Res digits, plus a decimal po indicator, and a 2-digit pos Results that exceed the digr Notation. Indicator Definition 2nd function. 2nd UVD Hunohaliarrow_forwardMiles per gallonarrow_forwardPlease answer Question 1arrow_forward
- What is the value of -12°F in units of °C? O :6.7 249 O -24 none of the above O 16arrow_forwardRounding Off Initial Number Student’s Rounded Value 1. Correct? (yes/no) 2. Corrected (if needed) 143.63212 144 532 800 533 0.008 583 45 0.009 8 8.00 Significant Figures in Calculations Multiplication and Division Perform the following multiplication and division calculations. Give a final answer with the correct number of significant figures: 0.1184 ´ 8.00 ´ 0.0345 (42.4)(15.6) 1.265 (35.56)(1.45) (4.8)(0.56) Addition and Subtraction Perform the following addition and subtraction calculations. Give a final answer with the correct number of decimal places. 13.45 mL + 0.4552 mL 145.5 m + 86.58 m + 1045 m 245.625 g – 80.2 g 4.62 cm – 0.885 cm…arrow_forwardPart A Carry out the calculation: (1.43)+(3.11) x (1.5) What is the answer rounded to the correct number of significant figures? View Available Hint(s) 06.1 6.8 06.0 6.095 Submitarrow_forward
- 4- Perform the following calculation and report the result to the correct number of significant figures: [29.3 – 23.1]/ 2.201] = (6.2) / (2.201) = 2.81690 Round to 2S.F. а) 3 b) 2.82 c) 2.817 d) 2.8 e) 2.816arrow_forwardUse the following made up equivalencies to answer the following questions: . 30.5q equals 1s 13.2s equals 1t . 82.1t equals 1p How many s are in 3.63x10²³t?arrow_forward= O MEASUREMENT Multiplication and division of measurements Decide whether each proposed multiplication or division of measurements is possible. If it is possible, write the result in the last column of the table. proposed multiplication or division (3.0 g)-(0.033 kg) = ? 3 (9.0 m²) - (8.0 m) = ? (3.0 kg). (1.0 m²) = ? Explanation Is this possible? Check Ⓒyes no Ⓒyes no yes no result 0 0 0 GOR ×10 X U 07 00 $ © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center |arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY