
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:=
O MEASUREMENT
Multiplication and division of measurements
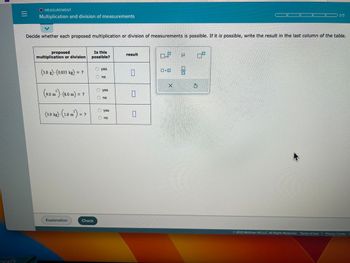
Decide whether each proposed multiplication or division of measurements is possible. If it is possible, write the result in the last column of the table.
proposed
multiplication or division
(3.0 g)-(0.033 kg) = ?
3
(9.0 m²) - (8.0 m) = ?
(3.0 kg). (1.0 m²) = ?
Explanation
Is this
possible?
Check
Ⓒyes
no
Ⓒyes
no
yes
no
result
0
0
0
GOR
×10
X
U 07
00
$
© 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center |
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Express each result in correct number of significant figures and with correct unit.arrow_forwardA bike has a 56 cm frame. What is this frame size equal to inches? (1.00 in = 2.54 cm. Not that this equivalence does not limit significant figures in the final answer)arrow_forwardCarry out the division and enter the quotient with the proper number of significant figures in scientific notation. (6.880 x 10-8) ÷ (0.348 x 10-3) =arrow_forward
- Decide whether each proposed multiplication or division measurements is possible. If it is possible, write the result in the last column of the table. proposed multiplication or division Is this possible? result 1.8 km O yes m = ? 300 6.0 s no |(20 cm')-(20 cm³) = ? O yes 8.0 cm no 60. cm yes = ? no 0.20 marrow_forwardHow many seconds are there in 3.7 years? (Use 365 days/year) (You do not need to put in your units & make sure to use the proper number of significant figures.arrow_forwardPerform the following calculations: a) 14.86 ml + 15.0 ml + 14.980 ml = b) (42.927 g/ml)(9.00 ml) = Round off the answers to the proper significant figures.arrow_forward
- = MEASUREMENT AND MATTER Setting up a unit conversion A student sets up the following equation to convert a measurement. (The ? stands for a number the student is going to calculate.) Fill in the missing part of this equation. Note: your answer should be in the form of one or more fractions multiplied together. g (75. 1/2) - = ² - ? mL x10 X L 9 CUBarrow_forwardBernard weighs a jar of pennies to find out how much they are worth. After subtracting the weight of the empty jar, he finds that the pennies have a total mass of 6.42 +/- 0.1 kg. Assuming that each penny weights 2.5 g, and taking into account the uncertainty of the measurement, what is the minimum value of the pennies (expressed in dollars)?arrow_forwardanswer in proper number of significant figures. 1. Convert 6.4 decades to seconds. 10 years)x(30 (24 hours 60 minuies 60 seconds I minatte 6.4 decades x 305 dags I decade I year I dag 1 hour 2. Convert 71.0 Pa to dyne/cm?. 3. It was found out that 2.0 kg of sucrose can dissolve per liter of water at 25°C. How many tablespoons of water is needed to dissolve 54 g of sucrose?arrow_forward
- ||| O MEASUREMENT AND MATTER Setting up a one-step unit conversion A student sets up the following equation to convert a measurement. (The ? stands for a number the student is going to calculate.) Fill in the missing part of this equation. 0.050 =).0 € 1 S 0x10 0.0 X μ 8 5arrow_forwardA cubic object has a side length of 3.17 cm. It is found to be a pure substance with a mass of 41.18 grams. What is the density of the substance in g/cm3? Numbers only, answer to 2 decimal places. Volume of cube = (Side)3arrow_forwardWhat is the volume (in mL) of (6.23x10^0) kg of a liquid that has a density of (1.1000x10^0) g/mL? Enter your answer in scientific notation with 3 sig figs. Do not include any units in your answer. Do not round any intermediate calculations. Note: Your answer is assumed to be reduced to the highest power possible. Your Answer:arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY