
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
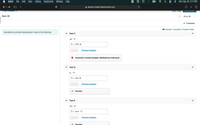
Need help on part C
Transcribed Image Text:Safari
File
Edit
View
History
Bookmarks
Window
Help
Mon Sep 28 3:14 PM
session.masteringchemistry.com
Test Chapter S
Hide Timer
Item 20
20 of 20
v Complete
II Review I Constants I Periodic Table
Calculate the unknown temperature
each
the following:
Part C
-24 °C
T = 273 K
%3D
Submit
Previous Answers
X Incorrect; correct answer withheld by instructor
Part D
31 °C
Т- 304 К
%3D
Submit
Previous Answers
Correct
Part E
103 °F
T = 39.4 °C
%3D
Submit
Previous Answers
Correct
00
Expert Solution
arrow_forward
Introduction
The temperature scale is used to measure degree of hotness or coldness of a body (or system). The SI unit of temperature is Kelvin (K).
Given data:
The temperature is –24oC.
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- S-3. CH3CH2C = CCH2CH3 → Yukarıdaki reaksiyonun ürünü nedir? 04 HgSH2SO4 H2O HgSO4/ H2SO4 CH3CH2CECCH2CH3 H₂O S-3. Yukarıdaki reaksiyonun ürünü nedir? ngarrow_forwardesc A common ketone starting material is shown below. Predict the major product for each reaction based on the reagents given. Ignore any inorganic byproducts. Select to Draw 1 I 1 CH3NH2, pH 4-5 9 16 > a 20 Select to Draw 1. NaBH4 2. H3O+ 000arrow_forward6. The following structure is a glycerophospholipid. a. Circle & label the 4 parts of the molecule: fatty acids (2), glycerol, phosphate, & amino alcohol. CH- O - C - (CH,),CH, 0 ...i. CH - 0 - C - (CHỊ),CH, CH₂-O-F CH₁ -CH₂-CH₂-N-CH, CH, b. Which part of the molecule is considered polar (hydrophilic)? (fatty acids, glycerol, or phosphate-amino alcohol)arrow_forward
- Select Draw Rings More Erase C H 1. СН-CH-MgBr 2. H3O*arrow_forwardhttps://drive.google.com/file/d/14UPPDWhJXm0KKcLAOXDyBULvI_sISzDW/view?usp=sharing Which hazard statement is false in regards to dichloromethane? 1) May be corrosive to metals 2) Causes skin irritation 3) Causes serious eye irritation 4) Suspected of causing cancerarrow_forwardExplain the model drawn. The video below may help you explain the model. Link: https://m.youtube.com/watch?v=y6uVmIYR07karrow_forward
- Which solvent(s) would you expect toluene to be miscible in? ооо Cyclohexane and ethanol Water and ethanol Cyclohexane Water and cyclohexanearrow_forwardسکہ Synthesis => => E1 => E2 => Wittig => Ph3P + glide ketone 2 Z + Brarrow_forwardComplete the following reactions by providing the missing information. а. OEt OEt diene dienophile b. Br2 (1 mole) CH2=CH-CH=CH2 -15°C (major product) (minor product) H3CO.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY