
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
9L.4
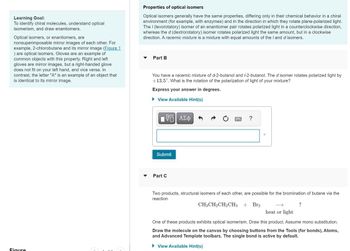
Transcribed Image Text:Learning Goal:
To identify chiral molecules, understand optical
isomerism, and draw enantiomers.
Optical isomers, or enantiomers, are
nonsuperimposable mirror images of each other. For
example, 2-chlorobutane and its mirror image (Figure 1
) are optical isomers. Gloves are an example of
common objects with this property. Right and left
gloves are mirror images, but a right-handed glove
does not fit on your left hand, and vice versa. In
contrast, the letter "A" is an example of an object that
is identical to its mirror image.
Figure
Properties of optical isomers
Optical isomers generally have the same properties, differing only in their chemical behavior in a chiral
environment (for example, with enzymes) and in the direction in which they rotate plane-polarized light.
The / (levorotatory) isomer of an enantiomer pair rotates polarized light in a counterclockwise direction,
whereas the d (dextrorotatory) isomer rotates polarized light the same amount, but in a clockwise
direction. A racemic mixture is a mixture with equal amounts of the I and d isomers.
Part B
You have a racemic mixture of d-2-butanol and /-2-butanol. The d isomer rotates polarized light by
+13.5°. What is the rotation of the polarization of light of your mixture?
Express your answer in degrees.
► View Available Hint(s)
VE ΑΣΦ
Submit
Part C
Two products, structural isomers of each other, are possible for the bromination of butane via the
reaction
CH3CH₂CH₂CH3 + Br₂
?
heat or light
One of these products exhibits optical isomerism. Draw this product. Assume mono substitution.
Draw the molecule on the canvas by choosing buttons from the Tools (for bonds), Atoms,
and Advanced Template toolbars. The single bond is active by default.
► View Available Hint(s)
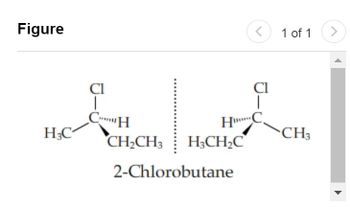
Transcribed Image Text:Figure
Cl
Jadian
CH
H₂C
H***
CH₂CH3 H3CH₂C
2-Chlorobutane
<
Cl
1 of 1
CH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 6. Consider the following chemical reaction: 2 Cr(OH)3(aq) >Cr₂O3(s) + 3H₂0(1) 12.00 moles of chromium (III) hydroxide is decomposed. Calculate moles of the water produced. O 18.00 moles O 1.500 moles O 4.00 moles O 12.00 molesarrow_forwardBecause of the toxicity of mercury compounds, mercury(I) chloride is used in antibacterial salves. The mercury(I) ion Hg22+ consists of two bound Hg+ ions.How many grams of mercury(I) chloride are needed to saturate 4900 km3 of water (the volume of Lake Michigan)? (Ksp = 1.5 × 10−18) .arrow_forwardConsider the following reactions:CoO (s) + CO (g) D CO2 (g) + Co (s) Kc(1) = 490.2 CoO (s) + 2 H2 (g) D 2 Co (s) + 2 H2O (g) Kc(2) = 4.5 x 103a. Write the overall equation for the reaction of hydrogen gas and carbon dioxide gas to produce carbon monoxide gas and steam.arrow_forward
- The great French chemist Antoine Lavoisier discovered the Law of Conservation of Mass in part by doing a famous experiment in 1775. In this experiment Lavoisier found that mercury(II) oxide, when heated, decomposed into liquid mercury and an invisible and previously unknown substance: oxygen gas. 1. Write a balanced chemical equation, including physical state symbols, for the decomposition of solid mercury(II) oxide (HgO) into liquid mercury and gaseous dioxygen. 0 2. Suppose 62.0 mL of dioxygen gas are produced by this reaction, at temperature of 130.0 °C and pressure of exactly 1 atm. Calculate the mass of mercury(II) oxide that must have reacted. Round your answer to 3 significant digits. g 010 X 0x12arrow_forwardThe great French chemist Antoine Lavoisier discovered the Law of Conservation of Mass in part by doing a famous experiment in 1775. In this experiment Lavoisier found that mercury(II) oxide, when heated, decomposed into liquid mercury and an invisible and previously unknown substance: oxygen gas. 1. Write a balanced chemical equation, including physical state symbols, for the decomposition of solid mercury(II) oxide (HgO) into liquid mercury and gaseous dioxygen. 2. Suppose 71.0 mL of dioxygen gas are produced by this reaction, at a temperature of 50.0 °C and pressure of exactly 1 atm. Calculate the mass of mercury(II) oxide that must have reacted. Be sure your answer has the correct number of significant digits.arrow_forwardA 14.599 g14.599 g sample of CaCl2CaCl2 was added to 12.147 g12.147 g of K2CO3K2CO3 and mixed in water. A 3.571 g3.571 g yield of CaCO3CaCO3 was obtained. What is the limiting reagent? CaCO3CaCO3 K2CO3K2CO3 CaCl2CaCl2 Calculate the percent yield of CaCO3.CaCO3. yield of CaCO3=CaCO3=arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY