
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Plz do Asap....!
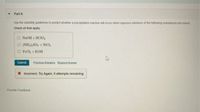
Transcribed Image Text:Part A
Use the solubility guidelines to predict whether a precipitation reaction will ocur when aqueous solutioris of the following substances are mixed
Check all that apply.
O NAOH + HCIO,
O (NH.),So, + NICI,
O FeCl, + KOH
Submit
Erevious Answers RequestAnswer
X Incorrect; Try Again; 6 attempts remaining
Provide Feedback
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- MALEKS T LI ALEKS - Rafia Riaz - Learn Type here to search www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-lvdw7xgLCkqMfg8yaFKbD9GafJstkYLIJnusptTd4BMWR5tqw8EEwiGTTdyzfqHFomOky9feFge9QQitSAMUOKacSVpwDeo?1... Q = O ELECTROCHEMISTRY Using the Nernst equation to calculate nonstandard cell voltage X + A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction: 2+ Sn²+ (aq) + Ba (s) → Sn (s) +Ba²+ (aq) 2+ 2+ Suppose the cell is prepared with 3.24 M Sn in one half-cell and 2.23 M Ba in the other. Calculate the cell voltage under these conditions. Round your answer to 3 significant digits. 0 Explanation 발 Check x10 ロ・ロ Olo 0/3 Rafia V ? 圖 □ □ 图 olo Ar © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility (?) 46°F Mostly clear * 9:09 AM 5/5/2023 x : Marrow_forwardI just need a little help on how to do this the explanations aren't helping me and I'm lost.arrow_forwardAsk Laftan Anlamaz - Episode St. John's University - My App X A ALEKS - Iffat Khan - Learn G In valence electrc -> A www-awn.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-lijkPWvZoZLgkt1FLIq7wcPWKzBYGfE9IMFj8s1-UE-m2k5S O MATTER Finding the side length of a cube from its volume in liters A technical machinist is asked to build a cubical steel tank that will hold 390 L of water. Calculate in meters the smallest possible inside length of the tank. Round your answer to the nearest 0.01 m. Omarrow_forward
- T LI ALEKS - Rafia Riaz - Knowledge CX b Answered: = Module Knowledge X + www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-lvdw7xgLCkqMfg8yaFKbD9GafJstkYLIJnusZsUfYJJOecqshL9ihmdkZd4zbXDleN-ax_luBKRpjrEFpTCwvorn6Uz9RRiR?1... Q (297) Yeh scene kuch samajh nai X Type here to search = Module Knowledge Check For each system listed in the first column of the table below, decide (if possible) whether the change described in the second column will increase the entropy S of the system, decrease S, or leave S unchanged. If you don't have enough information to decide, check the "not enough information" button in the last column. A few moles of nitrogen (N₂) gas. System A few moles of helium (He) gas. A few grams of ammonia vapor (NH₂). I Don't Know Et Submit Change The nitrogen is heated from -3.0 °C to 81.0 °C and is also compressed from a volume of 15.0 L to a volume of 9.0 L. The helium is compressed from a volume of 4.0 L to a volume of 3.0 L while the temperature is held constant at…arrow_forward2. Please deduce the following fundamental equations. TdS -PdV www du www =arrow_forwardn Course: CHEM262-SEC01 Org x V OWL Exam O Noor Abid - 32843659 S x b Success Confirmation of Quest x G how to take a screenshot on m + A owl.umass.edu/owlj/servlet/Student Update : M Gmail O YouTube O Google Keep n UMass Amherst M.. c Write The Comple... O SPIRE Logon A Swank Movies O Discovering Nutrit. 26 UMass Amherst -. t. Minitab >> Select the major product of the following reactions. 1. NaOEt 2. CH3I EtO OEt 3. NaOEt 4. CH3I 5. H,0*/ heat Is 6. CH,ОН/ Н+ HO, OH IV I II III O None of the choices O IV O IIarrow_forward
- 6:07 PM Wed Aug 4 * 63% learn-us-east-1-prod-fleet02-xythos.content.blackboardcdn.com + b My Questions |... Bb Homework – Or... Bb learn-us-east-1... Bb Presentations -... Bb Austin Commun... learn-us-east-1... Chem 2323 Org I Homework #18 H2O-I 1. What are the products and major product of each of the following reactions: а. hv + Cl2 b. hv + Cl2 с. hv HCI HBr hv + Нeat + NBS Peroxidearrow_forwardCan you please answer it , has been a hard subject for me . Thank youarrow_forward) 46% Sun 11:04 AM Safari File Edit View History Bookmarks Window Help A session.masteringchemistry.com Ellucian Degree Works... Pearson's MyLab & Mas... ALEKS - Sofia Simmons... MasteringChemistry: H... Consider summer class... Inbox (13) - thesym1@g.. Class Schedule Listing Schedule 111.009 & 111.... View Available Hint(s) Reset Help F2 H2 Mg O2 Oxidizing agent Reducing agent 31410 FEB tv 23 MacBook Pro esc 23 2$ & ....arrow_forward
- - (17 · (17 Fra Fra ge edu/courses/159861/files?preview=69941104 الولايات المتحدة القن... 0 Ns G Gmail als Science Problem Set 2.pdf W F2 # 2 ۲ 3 r b Ac E 80 F3 D 16 $ Why You Don't Ne... 4 E (PI ASU Fil W Juj (PL R Page of 2 | 0 | 9 al K Û F9 ) O PSU X 0 [ O A Alternative خ F10 . La P * Varrow_forward● A ALEKS-Lara AX → с www-awu.aleks.com/alekscgi/x/lsl.exe/10_u-IgNslkr7j8P3jH-IvTqeviKFP6W0cqJcWJdIACROQwyw24GWHIntrzjVfiaJ_cKvcyqWTUEIAKXtcoqhtE9_vix... 4 Solubility and... 18.3 Gibbs Free E... 5.3 Enthalpies of.... 18.5 Gibbs Free E... Reading Schedule 19.6 Reduction Po... C ! 1 Under these conditions, calculate the reaction free energy AG for the following chemical reaction: N₂(g) + 3H₂(g)2NH₂(g) Use the thermodynamic information in the ALEKS Data tab. Round your answer to the nearest kilojoule. ● ENTROPY AND FREE ENERGY Calculating reaction free energy under nonstandard conditions Explanation A T A chemist fills a reaction vessel with 7.76 atm nitrogen (N₂) gas, 7.51 atm hydrogen (H₂) gas, and 0.524 atm ammonia (NH₂) gas at a temperature of 25.0°C N Puppy Jobs, Em x G puppy store ne x Part Time Jobs, x G Food Server and X G 10 out of 25 as x) How Many Hour x CO @ 2 Check W S X R option command #3 X E D 26 $ DS 4 C Ś R LL F % 5 V T tv SH N G MacBook Pro ^ 6 Y B & M 7 19.6 Reduction…arrow_forwardMALEKS T LI ALEKS - Rafia Riaz - Knowledge CX + www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-lvdw7xgLCkqMfg8yaFKbD9GafJstkYLIJnusWtHPKeXbFh_esrw9lm-BbWISQqFfd1OKKzQ55nmjVf-6W1kBQ5aycVAYBaQo?... Type here to search = Module Knowledge Check Read the descriptions of physical or chemical changes in the table below. Then decide whether the change will be spontaneous, if you can. A chemical reaction between two liquids creates gaseous products. A gas expands, absorbing heat from its surroundings. Change During an exothermic chemical reaction, a solid is consumed and a gas produced. I Don't Know Et Submit Question 1 3 Is this change spontaneous? Yes. O No. O Can't decide with information given. O Yes. O No. O Can't decide with information given. O Yes. O No. O Can't decide with information given. X Rafia V ? 13 ollo © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility 65°F Mostly clear (?) F * (P 9:08 PM 5/10/2023 x : 6arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY