
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Part A
Recall all the models you described in task 1. Think about the results each model would predict for the experiment with hydrogen gas. Which models of the atom does the experimental evidence support? Explain why these models are compatible with the experimental results.
Part B
Which models of the atom in task 1 are not supported by the results of the hydrogen gas experiment? For each of these models, explain the experimental results that the model would predict.
Models in task 1:
- Dalton’s Atomic Model
- Thomson’s Atomic Model
- Rutherford’s Atomic Model
- Bohr’s Atomic Model
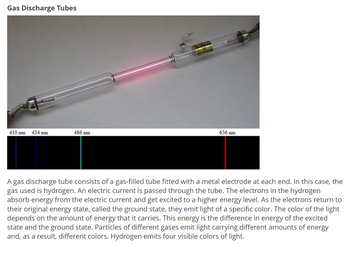
Transcribed Image Text:Gas Discharge Tubes
410 nm 434 nm
486 nm
656 nm
A gas discharge tube consists of a gas-filled tube fitted with a metal electrode at each end. In this case, the
gas used is hydrogen. An electric current is passed through the tube. The electrons in the hydrogen
absorb energy from the electric current and get excited to a higher energy level. As the electrons return to
their original energy state, called the ground state, they emit light of a specific color. The color of the light
depends on the amount of energy that it carries. This energy is the difference in energy of the excited
state and the ground state. Particles of different gases emit light carrying different amounts of energy
and, as a result, different colors. Hydrogen emits four visible colors of light.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What if Rutherford believed atoms were as Dalton envisioned them? What do you suppose Rutherford would have expected, and what would have surprised him?arrow_forwardHow many electrons, protons, and neutrons are contained in each atom?a. gallium-69 c. titanium-48b. fluorine-23 d. tantalum-181arrow_forwardWas Democritus’s proposal of the existence of atoms based on scientific methods or ideas? Explainarrow_forward
- Which chemical reactions are not possible according to Dalton's atomic theory? CC14 → CH4 N₂ + 3H₂ 2H₂ + O₂ 2 2NH3 2 H₂O + Auarrow_forwardNeed help with this pjhysics theoretical question: The most surprising evidence from Rutherford’s experiment that disproved Thomson’s model was that: the alpha particles were able to pass through the gold foil most of the alpha particles were deflected by less than one degree a few alpha particles were scattered through very large angles the scattering was less random than expectedarrow_forwardng tu another question will save this response. stion 2 Which part(s) of Dalton's atomic theory were demonstrated to be incomplete a. Matter is made of indivisible atoms O b. Atoms are made of protons, neutrons, and electrons C. Each atom is identical to others of the same element d. Atoms of a specific element are different from atoms of all other element e. Some atoms of the same elements can combine in different whole numbe Of. Atoms combine in whole number ratios to form compoundsarrow_forward
- 2. Which of the following best describes an element? a. A neutral substance consisting of a cation and an anion b. A substance consisting of two non-metals C. A substance consisting only of atoms having the same nur of protons. d. A substance that can only be found as a solid e. A substance consisting only of atoms having the same num of neutrons O a O b O c dlarrow_forwardAtomic Theory & Scientists 1. Write the letter of each sentence that is true about Dalton's atomic theory. a. All elements are composed of tiny, indivisible particles called atoms. An element is composed of several types of atoms. b. c. Atoms of different elements can physically mix together, or can chemically combine in simple, whole-number ratios to form compounds. d. Chemical reactions occur when atoms are separated, joined or rearranged; however, atoms of one element are never changed into atoms of another element by a chemical reaction.arrow_forwardWhich subatomic particle is transmitted through electric current? A. proton B. electron C. electron D. nucleusarrow_forward
- Which of John Dalton’s theories given below were found to be INCORRECT?1. the atoms of elements remain unchanged when they combine to form chemical compounds2. the atom is the smallest unit of matter that retains its chemical identity3. all atoms of the same element are identical4. the atom cannot be divided into any smaller unit of massA) 2 and 3 D) 1, 2, and 3B) 3 and 4 E) all four were found to be correct C) 1,3,and4arrow_forward6. Who discovered the nucleus of the atom? A. Dalton B. Rutherford C. Millikan D. Thomson 7. The five spheres below represent Se, Se2-, O, H, and S, not necessarily in that order. 37 pm 72 pm 100 pm 114 pm 196 pm Which answer correctly orders all five species from smallest to largest? A. O х"(g) + e B. X*(g) → X*(aq) С. Х(g) + e -> X (з) D. X*(g) + e→ X(g) 9. Which of the following compounds are insoluble? A. Pbl2 В. CaSO4 С. CSCI D. Nazs E. Both A and B 10. Assign oxidation numbers to the chlorine atom in each of the following compounds: Cl2, HCI, ClO3, CIF3, PCI3 А. О, —1, +6, +3, -1 В. О, —1, +5, +3, -3 С. 0, —1, +5, +3, —1 D. 0, -1, +3, +3, –1arrow_forwardIf you wanted to make an accurate scale model of the hydro- gen atom and decided that the nucleus would have a diameter of 1 mm, what would be the diameter of the entire model? I understand the answer however wouldnt the end result be much smaller than 106 mm since we are only find the diameter of the nucleus? Is the 106 always used in these types of equations? What would be used in this equation for say a carbon atom?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY