
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
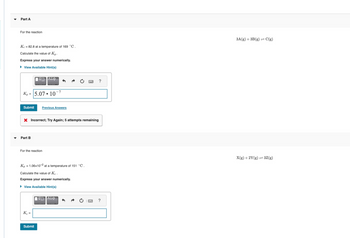
Transcribed Image Text:Part A
For the reaction
Kc = 82.8 at a temperature of 169 °C.
Calculate the value of Kp.
Express your answer numerically.
▸ View Available Hint(s)
Kp 5.07.10
Submit
Part B
IVD ΑΣΦ
|
X Incorrect; Try Again; 5 attempts remaining
Previous Answers
For the reaction
K₂ =
Kp = 1.06x10-2 at a temperature of 151 °C.
Calculate the value of Ke.
Express your answer numerically.
► View Available Hint(s)
ΑΣΦ
Submit
?
?
3A(g) + 3B (g) = C(g)
X(g) + 2Y(g) =3Z(g)

Transcribed Image Text:The equilibrium constant, K., is calculated using molar concentrations. For gaseous reactions another form of the equilibrium constant, Kp, is
calculated from partial pressures instead of concentrations. These two equilibrium constants are related by the equation
K₂ = K. (RT)An
where R = 0.08206 L-atm/(K-mol), T is the absolute temperature, and An is the change in the number of moles of gas (sum moles
products - sum moles reactants). For example, consider the reaction
N₂(g) + 3H₂(g) = 2NH3(g)
for which An=2-(1+3) = -2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Mercury(IT) oxide decomposes to form mercury and oxygen, like this: 2HgO(s)--2Hg(l)+O2(g) At a certain temperature, a chemist finds that a 3.5 L reaction vessel containing a mixture of mercury (It) oxide, mercury, and oxygen at equilibrium has the following composition: compound amount HgO 15.3 g Hg 19.1 g O2 25.0 g Calculate the value of the equilibrium constant K, for this reaction, Round your answer to 2 significant digits.arrow_forwardAa.49.arrow_forward7.B Consider the chemical equation C(s) + 2A(g) 5 2B(I) + heat. Be sure to briefly JUSTIFY each answer below. (a) Will SQUEEZING the container cause a disturbance to equilibrium? If so, then in what direction will the reaction occur? (b) Will RAISING THE TEMPERATURE of the system cause a disturbance to equilibrium? If so, then in what direction will the reaction occur? (c) What is the sign of the standard reaction ENTHALPY? Does the enthalpy change provide a favorable or unfavorable contribution to the spontaneity of the forward reaction at standard state conditions? (d) What is the sign of the standard reaction ENTROPY? Does the entropy change provide a favorable or unfavorable contribution to the spontaneity of the forward reaction at standard state conditions? (e) Based on (c,d) describe the preference for products or reactants at equilibrium at very LOW temperatures. (f) Based on (c,d) describe the preference for products or reactants at equilibrium at very HIGH temperatures.arrow_forward
- A sample of solid (NH4)(NH2CO2) is placed in an evacuated container at 25°C, and it decomposes as follows with K = 231x10 (NH4)(NH>CO2)(0) 2 NH3(g) + CO2(g) Which aspoct of this reaction at this temperature cannot be determined based on the information provided in the question? Selec one O a the sign of As° for thc reaction b. the sign of AG° for the reaction Oc the magnitude of the heat flow (q) for the reaction Od the sign of the heat flow (g) for the reaction O e the sign of the work (w) for the reaction 1 the magnitude of AG° for the reactionarrow_forwardT4.15 The equilibrium constant (K.) for the reaction 2HCI(g) = H2(g) + Cl2(g) is 4.17 x 10-34 at 25°C. What is the equilibrium constant for the reaction H2(g) + Cl2(8) = 2HCI(g) at the same temperature?arrow_forward= The equilibrium system between sulfur dioxide gas, oxygen gas, and sulfur trioxide gas is given. 2 SO₂(g) + O₂(g) 2 SO3(g) Write the balanced chemical equation for the reverse reaction. Include physical states for all species. chemical equation: 20 R F de 10 G Search or type URL % 5 70 V T B tv 6 MacBook Pro Y H & M 7 N U J 00 8 M A I ( 0. 9 K < O < H -C O L ») P • V | 1 : { [ I command option = O ? 1 deletarrow_forward
- Nitrogen and hydrogen react to form ammonia, like this: N₂(g) + 3 H₂(g) → 2NH3(g) Also, a chemist finds that at a certain temperature the equilibrium mixture of nitrogen, hydrogen, and ammonia has the following composition: compound pressure at equilibrium N₂ 67.1 atm H₂ NH3 K = P 43.5 atm Calculate the value of the equilibrium constant K for this reaction. Round your answer to 2 significant digits. р 0 83.8 atm x10 X Śarrow_forwardplease show steps, thanksarrow_forward1/5 Ammonia will decompose into nitrogen and hydrogen at high temperature. An industrial chemist studying this reaction fills a 5.0L flask with 1.0 atm of ammonia gas, and when the mixture has come to equilibrium measures the amount of nitrogen gas to be 0.25 atm. Calculate the pressure equilibrium constant for the decomposition of ammonia at the final temperature of the mixture. Round your answer to 2 significant digits. K, = 0 %3D x10 alo Ar Explanation Check 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center Acces 山國 TO00 21 ? FEB tv 23 MacBook Air DII DD 888 F10 F9 esc F6 F6 F7 F1 F2 F3 F4 %23 2$ 5 7 8 1 W E Y Tarrow_forward
- Using any data you can find in the ALEKS Data resource, calculate the equilibrium constant x at 25.0 °c for the following reaction. N₂(g) + 3H₂(g) 2 NH3(g) Round your answer to 2 significant digits. K = 1 0 x10 Xarrow_forwardConsider the reaction: SO₂(g) + 1/2O2(g) ⇒ SO3 (9) Write the equilibrium constant for this reaction in terms of the equilibrium constants, K₁ and K2, for the reactions below: 2S(s) + 30₂ (g)2SO3 (9) K₁ S(s) + O₂(g) ⇒ SO₂(g) K₂ For answers with both a subscript and a superscript, enter the subscript first. For example, enter Kif the first equilibrium constant should be squared. K =arrow_forward21. Please refer to the attached photo. THank youuuarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY