
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:=
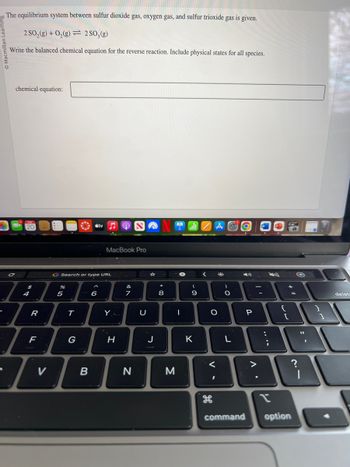
The equilibrium system between sulfur dioxide gas, oxygen gas, and sulfur trioxide gas is given.
2 SO₂(g) + O₂(g) 2 SO3(g)
Write the balanced chemical equation for the reverse reaction. Include physical states for all species.
chemical equation:
20
R
F
de 10
G Search or type URL
%
5
70
V
T
B
tv
6
MacBook Pro
Y
H
&
M
7
N
U
J
00
8
M
A
I
(
0.
9
K
<
O
<
H
-C
O
L
»)
P
• V
| 1
:
{
[
I
command option
=
O
?
1
delet
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Suppose a 500. mL flask is filled with 1.6 mol of H₂ and 0.30 mol of HI. This reaction becomes possible: H₂(g) + 12₂(g) → 2HI(g) Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the equilibrium molarity of each compound after the reaction has come to equilibrium. Use x to stand for the unknown change in the molarity of I2. You can leave out the M symbol for molarity. initial change equilibrium H₂ 0 1₂ x 0 HI 0 X Sarrow_forwardSuppose a 250. mL flask is filled with 0.60 mol of SO₂ and 1.0 mol of SO3. This reaction becomes possible: 2SO₂(g) + O₂(g) — 2SO3(g) Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the equilibrium molarity of each compound after the reaction has come to equilibrium. Use x to stand for the unknown change in the molarity of O₂. You can leave out the M symbol for molarity. initial change equilibrium SO₂ 0₂ 0 X 0 SO₂ 0 010 X Śarrow_forwardSuppose a 500. mL flask is filled with 0.10 mol of O, and 1.3 mol of SO3. This reaction becomes possible: 3' 2S0,(g) + 02(g) =2S03(g) Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the equilibrium molarity of each compound after the reaction has come to equilibrium. Use x to stand for the unknown change in the molarity of O,. You can leave out the M symbol for molarity. so, O2 so, initial change equilibriumarrow_forward
- Consider the following system at equilibrium: CH4(g) + H2O(g) D CO (g) + 3H2 (g) ΔH° = +206 kJ. Which of the following changes will shift the equilibrium to the left? decreasing the pressure adding a catalyst adding methane increasing the temperature decreasing the volumearrow_forwardWhat is the correct equilibrium constant expression for the balanced reaction shown below? 2 HCl (g) + I2 (s) 2 HI (g) + Cl2 (g)arrow_forwardWhich of the following statements is a true statement concerning a reaction that has reached a state of equilibrium? A system has reached equilibrium when the concentrations of reactants and products remain constant. A system has reached equilibrium when the reaction has stopped and no more products are formed. A system has reached equilibrium when the rate constant for the forward reaction equals the rate constant of the reverse reaction. A system has reached equilibrium when the concentrations of reactants and products correspond to the stoichiometric ratios determined by the balanced equation.arrow_forward
- The equilibrium system between hydrogen gas, bromine gas, and hydrogen bromide gas is given. H, (g) + Br, (g) = 2 HBr(g) Write the balanced chemical equation for the reverse reaction. Include physical states for all species. chemical equation:arrow_forwardGiven the following equation: NH4 (aq) + NO2 (aq) → N2(g) + 2H2O (1) AH = -122.4 kJ/mol How would decreasing the pressure effect the equilibrium? It would cause the equilibrium to shift to the right, favoring the products or the forward reaction. It would cause the equilibrium to shift to the left, favoring the reactants or the reverse reaction. It would not effect the equilibrium.arrow_forwardSuppose a 250. mL flask is filled with 1.6 mol of I, and 1.3 mol of HI. This reaction becomes possible: H,(g) +I,(g) = -2HI(g) Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the equilibrium molarity of each compound after the reaction has come to equilibrium. Use x to stand for the unknown change in the molarity of H,. You can leave out the M symbol for molarity. H, I, HI initial change equilibrium olo Xarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY