
Chemistry: Principles and Reactions
8th Edition
ISBN: 9781305079373
Author: William L. Masterton, Cecile N. Hurley
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
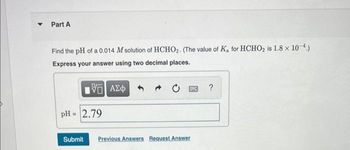
Transcribed Image Text:Part A
Find the pH of a 0.014 M solution of HCHO₂. (The value of K, for HCHO₂ is 1.8 x 10-4.)
Express your answer using two decimal places.
15. ΑΣΦ
pH = 2.79
Submit
ISHIC
Previous Answers Request Answer
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The pH of a 0.10-M solution of caffeine is 11.16. Determine Kb for caffeine from these data: C8H10N4O2(aq)+H2O(l)C8H10N4O2H+(aq)+OH(aq)arrow_forwardUsing the diagrams shown in Problem 10-37, which of the four acids is the weakest acid?arrow_forwardAre solutions of the following salts acidic, basic, or neutral? For those that are not neutral, write balanced chemical equations for the reactions causing the solution to be acidic or basic. The relevant Ka and Kb values are found in Tables 13-2 and 13-3. a. NaNO3 b. NaNO2 c. C5H5NHClO4 d. NH4NO2 e. KOCl f. NH4OClarrow_forward
- Find [OH+], [OH-] and the pH of the following solutions. (a) 30.0 mL of a 0.216 M solution of HCI diluted with enough water to make 125 mL of solution. (b) A solution made by dissolving 275 mL of HBr gas at 25C and 1.00 atm in enough water to make 475 mL of solution. Assume that all the HBr dissolves in water.arrow_forwardTwo strategies are also followed when solving for the pH of a base in water. What is the strategy for calculating the pH of a strong base in water? List the strong bases mentioned in the text that should be committed to memory. Why is calculating the pH of Ca(OH)2 solutions a little more difficult than calculating the pH of NaOH solutions? Most bases are weak bases. The presence of what element most commonly results in basic properties for an organic compound? What is present on this element in compounds that allows it to accept a proton? Table 13-3 and Appendix 5 of the text list Kb values for some weak bases. What strategy is used to solve for the pH of a weak base in water? What assumptions are made when solving for the pH of weak base solutions? If the 5% rule fails, how do you calculate the pH of a weak base in water?arrow_forwardConsider 0.10 M solutions of the following compound: AlCl3, NaCN, KOH, CsClO4, and NaF. Place these solutions in order of increasing pH.arrow_forward
- Calculate [OH-] and pH in a solution in which the hydrogen sulfite ion, HSO3-, is 0.429 M and the sulfite ion is (a) 0.0249 M (b) 0.247 M (c) 0.504 M (d) 0.811 M (e) 1.223 Marrow_forward. Consider 0.25 M solutions of the following salts: NaCl. RbOC1, KI, Ba(ClO4),, and NH4NO3. For each salt, indicate whether the solution is acidic, basic, or neutral.arrow_forwardAre solutions of the following salts acidic, basic, or neutral? For those that are not neutral, write balanced equations for the reactions causing the solution to be acidic or basic. The relevant Ka, and Kb values are found in Tables 13-2 and 13-3. a. Sr(NO3)2 b. NH4C2H3O2 c. CH3NH3Cl d. C6H5NH3ClO2 e. NH4F f. CH3NH3CNarrow_forward
- The following illustration displays the relative number of species when an acid, HA, is added to water. a. Is HA a weak or strong acid? How can you tell? b. Using the relative numbers given in the illustration, determine the value for Ka and the percent dissociation of the acid. Assume the initial acid concentration is 0.20 M.arrow_forwardA solution of baking soda, NaHCO3, has a pH of 10.08. What is the percent (by mass) of NaHCO3 in a 235-mL solution? (Assume a density of 1.00 g/mL.)arrow_forwardWhat is a salt? List some anions that behave as weak bases in water. List some anions that have no basic properties in water. List some cations that behave as weak acids in water. List some cations that have no acidic properties in water. Using these lists, give some formulas for salts that have only weak base properties in water. What strategy would you use to solve for the pH of these basic salt solutions? Identify some salts that have only weak acid properties in water. What strategy would you use to solve for the pH of these acidic salt solutions? Identify some salts that have no acidic or basic properties in water (produce neutral solutions). When a salt contains both a weak acid ion and a weak base ion, how do you predict whether the solution pH is acidic, basic, or neutral?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
