
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
please help with all parts of this question. DOUBLE CHECK YOUR ANSWERS AS PREVIOUS TUTORS GOT IT WRONG.
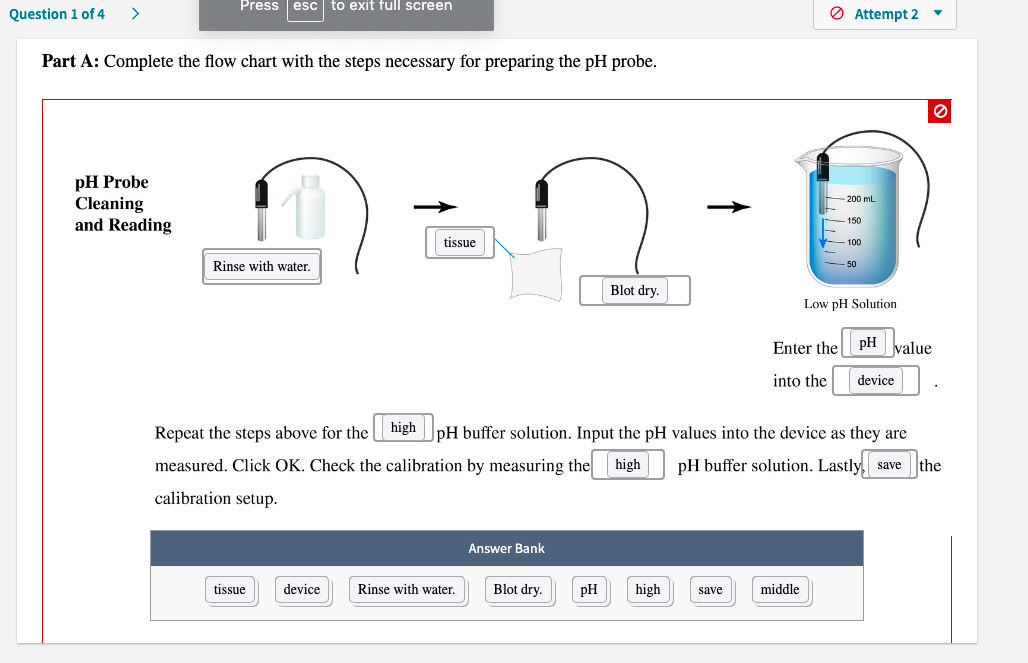
Transcribed Image Text:Part A: Complete the flow chart with the steps necessary for preparing the pH probe.
pH Probe
Cleaning
and Reading
200 mL
150
| tissue
100
Rinse with water.
50
Blot dry.
Low pH Solution
Enter the pH value
into the
device
Repeat the steps above for the high |pH buffer solution. Input the pH values into the device as they are
measured. Click OK. Check the calibration by measuring the high
pH buffer solution. Lastly, save the
calibration setup.
Answer Bank
tissue
device
Rinse with water.
Blot dry.
pH
high
middle
save
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1. What are fibers and where are they found? How could forensic scientists use fibers to solve a crime? 2. Define the word probative. What is the probative value of a fiber in a forensic investigation? 3. Describe steps you would take from collecting a fiber(s) at a crime scene to performing an analysis to determine its identity. 4. Which of the 6 fiber types you observed are natural? Which are man-made? 5. Are fibers classified or individualized? Why?arrow_forward3arrow_forwardI know you don't answer graded questions and I am not looking for you to straight up give me the answer to this but how do I even start? like I said before I know that the highest peak is 16amu which is Oxygen but do I just the vertical bar as a ratio? Like the next bar to the left (15amu?) is like 90% intensity? so like 9:10 ratio? There isn't even a 15amu element so please just give me some idea where to start. The place in the book it tells you to go to read has nothing about this stuff, its about finding empirical formulas from mass or percentages.... please help me!arrow_forward
- 6. Suppose you have a mixture of water and your 2-bromo-2-methylbutane product in a separatory funnel. Use densities to predict which phase will be the top layer in the funnel. a. 2-bromo-2-methylbutane (organic phase) b. water (aqueous phase) c. there would only be one phase since the substances are misciblearrow_forwardConsider an oxygen atom contained in a neutral molecule, like those in Model 1. Give the name for the electrons around the oxygen that are not involved in a covalent bond. What bond angle is formed when oxygen forms two single bonds? What shape is formed? Which functional groups in Model 1 are polar? When a carbon atom forms a double bond to an oxygen atom, it is called a carbonyl group. Which functional groups in Model 1 contain a carbonyl group?arrow_forwardWhy are ducks waterproof? It’s because they produce copious amounts of oils from their uropygial glands and spread it across their feathers. In this exercise, we’ll be investigating the molecular structure of one of these preen oils to determine how it keeps ducks dry. Q.5 - Preen oil is actually a complicated mixture of many different organic compounds, such as the structure seen previously.. Ornithologists have determined that birds often use preen oil compounds for scent recognition. Below, several different chemicals isolated from preen oil are shown, along with their vapor pressures at room temperature. p-cymene has the highest vapor pressure, meaning it is the most easily evaporated compound of the three listed. Explain why p-cymene has a higher vapor pressure at room temperature compared to the other compounds. Make sure to explain what holds the p-cymene in the sample. (Image attached)arrow_forward
- Please answer questions 3 and 4 and show work please. Thank youarrow_forwardThe above solution was wrong when entered into homework sitearrow_forwardBased on a grade 11 chemistry student, answer the following question based on the provided table:Part B: Other Cases EmergeRead Case File 3. Based on these new cases, it now seems clear that people are being poisoned by something in the Tylenol. Fast-Acting PoisonsWhatever the poison is, it is very fast acting as victims collapsed within minutes of taking the pills. After some research, and based on your love of Agatha Christie novels, you find five common poisons that work quickly and can cause death within a few hours – aconite, coniine, cyanide, nicotine, strychnine. Use the information contained in table 6 to determine the empirical formula, molecular formula, and fatal volume for the two poisons not completed in the table.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY