
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
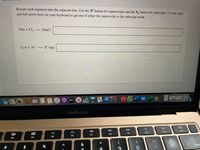
Transcribed Image Text:Retype each equation into the adjacent box. Use the X' button for superscripts and the X, button for subscripts. Use the right
and left arrow keys on your keyboard to get out of either the superscript or the subscript mode.
2Na + Cl,
2NaCl
L(s) + 2e 21¯(aq)
W
étv
26
MacBook Air
DII
DD
F12
F11
F10
F9
80
F8
F7
F6
F5
F3
F4
*
&
dele
%23
$
9
く
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Fill in the missing information for each of the 4 reactionsarrow_forward10 Homework • Unanswered CO2(8) HCl(aq) + _Na2C03(aq) →_NaCl(aq)+_CO2(e) + _H2O(1) Identify the net ionic equation. Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. 2H*(aq)+ CO (aq)→CO2(g)+H2O(1) a b H*(aq)+ CO-(aq)-CO2(g)- H20(1I) 2Na+ (aq)+2CI¯(aq)→2NaCl(s) d 2H+ (aq)+ 2Cl-(aq)→2HCI(s)arrow_forwardAnswer choices for blank 1(there is only one correct answer for each blank): A B C D E F G H J Answer choices for blank 2(there is only one correct answer for each blank): A B C D E F G H Jarrow_forward
- What is the product of the following reaction?arrow_forwardFor the acid-base reaction of sodium bicarbonate, NaHCO3, with hydrochloric acid, HCl, select all of the products of the reaction For the acid-base reaction of sodium bicarbonate, NaHCO3, with hydrochloric acid, HCl, select all of the products of the reaction NaCl NaOH OH- Na2CO3 H2O CO2 CO32- H+arrow_forwardb My Questions | bartleby rellum.ecollege.com/course.html?courseld%316519516&OpenVellumHMAC=Da14e8ada06537be6b0 Best Free PowerP... L Google Drive Academic Search I Downloads ouTube Maps Submit Request Answer Part G Write the formula of the conjugate acid for base Br. Express your answer as a chemical formula or an ion. NA chemical reaction does not occur for this question. Submit Request Answer Part H Write the name of the conjugate acid found in part G. Spell out the full name of the compound. Submit Request Answerarrow_forward
- A 2.5 M solution of a weak acid is prepared. Using a pH meter, the pH is measured to be 5.1. Calculate the acid ionization constant, Ka, of this weak acid. Show your work.arrow_forwardIn each row check off the boxes that apply to the highlighted reactant. reaction + Ag+ (aq) + 2 NH3(aq) → Ag(NH3)2(aq) H₂(g) + Br₂(g) → 2 HBr(g) HNO₂(aq) + C₂H₂NH₂(aq) → NO₂(aq) + C₂H₂NH₂(aq) The highlighted reactant acts as a... (check all that apply) Brønsted-Lowry acid Brønsted-Lowry base Lewis acid Lewis base Brønsted-Lowry acid Brønsted-Lowry base Lewis acid Lewis base Brønsted-Lowry acid Brønsted-Lowry base Lewis acid Lewis basearrow_forwardClassify each of the 3 reactions. Thank you!arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY