
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
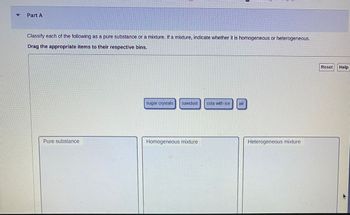
Transcribed Image Text:Part A
Classify each of the following as a pure substance or a mixture. If a mixture, indicate whether it is homogeneous or heterogeneous.
Drag the appropriate items to their respective bins.
Pure substance
sugar crystals sawdust cola with ice Eur
Homogeneous mixture
Heterogeneous mixture
Reset Help
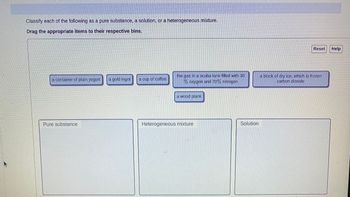
Transcribed Image Text:Classify each of the following as a pure substance, a solution, or a heterogeneous mixture.
Drag the appropriate items to their respective bins.
a container of plain yogurt a gold ingot
Pure substance
a cup of coffee
the gas in a scuba tank filled with 30
% oxygen and 70% nitrogen
a wood plank
Heterogeneous mixture
Solution
Reset
a block of dry ice, which is frozen
carbon dioxide
Help
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How do we know a substance is a compound, mixture or element?arrow_forwardDecide whether each sketch shows a pure sample of an element or a pure sample of a compound. R Substance A 48 P element compound * Substance C element compound * Substance B 9 8989 O element compound Substance D element compoundarrow_forwardA piece of metal with a mass of 114 g was placed into a graduated cylinder that contained 25.00 mL of water, raising the water level to 42.50 mL. What is the density of the metal? 7.25 g/cm³ 2.68 g/cm³ • 6.51 g/cm³ Which of the following is an example of a heterogeneous mixture? • iron • tin can 0.154 g/cm³ 0.592 g/cm³ brass gravel uranium How many cubic centimeters of ore containing 0.22% by mass gold must be processed to obtain $100 worth of gold? The density of the ore is 8.0 g/cm³ and the price of gold is $418 per troy ounce. (14.6 troy oz = 1.0 ordinary pound, called an avoirdupois pound; 1 lb = 454 g) 9.3 x 10¹ cm3³ • 4.2 x 10² cm³ • 6 200 cm³ 42 cm³ • 2.7 x 104 cm³arrow_forward
- Determine whether each change is physical or chemical.arrow_forwardWhich of the following is an example of a chemical property? O iron easily oxidizes O water will boil at 100 degrees O gold is yellow and shiny O table salt is soluble in water a hp osing Autod IIarrow_forwardIf matter is uniform throughout, cannot be separated into other substances by physical processes, and cannot be decomposed into other substances by chemical processes, it is: Group of answer choices a heterogeneous mixture a compound a mixture of compounds an element a homogeneous mixturearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY