
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
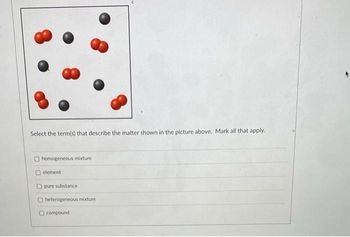
Transcribed Image Text:Select the term(s) that describe the matter shown in the picture above. Mark all that apply.
homogeneous mixture
element
pure substance
heterogeneous mixture
compound
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- potato salad salt water white wine bronze gravel Homogenous mixture Heterogeneous mixturearrow_forwardIdentify each of the following: (As: element, homogeneous mixture, heterogeneous mixture, compound, physical change, chemical property, physical property, heterogeneous mixture, homogeneous mixture, or chemical change.) Sodium nitrite preservative Mercury in thermometer Copper and rust Burning a log of wood Jet fuel Melting copper Chewing on a piece of bread Ibuprofen The process of sweating Cookies and cream ice cream Platinum ring Propane Graphite (a form of carbon) Densityarrow_forwardWhich state of matter will always fill always fill the volume and shape of the container in which it is placed?arrow_forward
- How are the properties of a compound related to those of the elements that comprise it?arrow_forwardWhich of the following is a pure substance that can be broken down into simpler substances by chemical means? Solution Compound Heterogeneous mixturearrow_forwardHow do we know a substance is a compound, mixture or element?arrow_forward
- Choose the homogeneous mixture from the list below. O soda (pop) air concrete trail mix bloodarrow_forwardDecide whether each sketch shows a pure sample of an element or a pure sample of a compound. R Substance A 48 P element compound * Substance C element compound * Substance B 9 8989 O element compound Substance D element compoundarrow_forwardGasoline is composed of a variety of different liquid hydrocarbons, which do not separate as time passes. Gasoline is an example of a: A) heterogeneous mixture B) Chemical compound C) Chemical element D) Solutionarrow_forward
- is sugar water considered to be a heterogenous mixture and if so whyarrow_forwarddescription of an element at the particulate levelarrow_forward2nd - Which of the following is false for mixtures? a) Mixtures have the properties of the substances that make them up. B) Sandy water is an example of a heterogeneous mixture. NS) Cologne is an example of a homogeneous mixture. D) Mixtures are separated into their components by chemical method. TO) Mixtures are divided into homogeneous and heterogeneous.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY