
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
K
Please don't provide the handwriting solution
Transcribed Image Text:▼
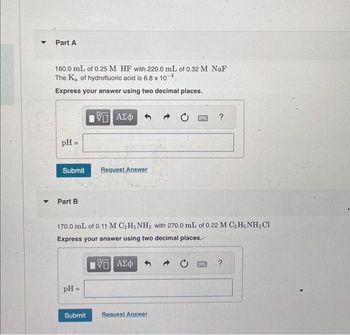
Part A
160.0 mL of 0.25 M HF with 220.0 mL of 0.32 M NaF
The Ka of hydrofluoric acid is 6.8 x 10-4.
Express your answer using two decimal places.
pH =
Submit Request Answer
Part B
15 ΑΣΦ
pH =
170.0 mL of 0.11 M C₂H5NH2 with 270.0 mL of 0.22 M C₂H5NH3 Cl
Express your answer using two decimal places.
ΨΕ ΑΣΦ
Submit
Request Answer
→ C
?
F
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 9 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Assuming that the standard solution contains 20.0 g/L of Red Dye #40, report the concentration in g/L of Red Dye #40 in your unknown. The path length is 1.00cm To test the unknown and standard solution, 0.1 mL of the unknown was dispensed into a 50-mL volumetric flask about three-fourths full with deionized water. this was done 1 more time for the unknown and two times for the standard solution. Therefore at the end, there were 4 flasks each with 0.1 mL of sample (2-unknown and 2-standard solution).arrow_forwardI have no idea how to do the rest please helparrow_forwardWhat goal is accomplished by standardizing solutions?arrow_forward
- Xylometazoline hydrochloride paediatric nasal drops are formulated as a 0.05% w/v solution. What amount of xylometazoline hydrochloride is present in 10 mL of solution?A) 1 mgB) 5 mgC) 10 mgD) 50 mgE) 100 mgarrow_forwardClick in the answer box to display choices. Predict the water solubility of the following organic molecule: OH carotatoxin (neurotoxin isolated from carrots) (select)arrow_forwardPlease don't provide handwrittin solution....arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY