
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
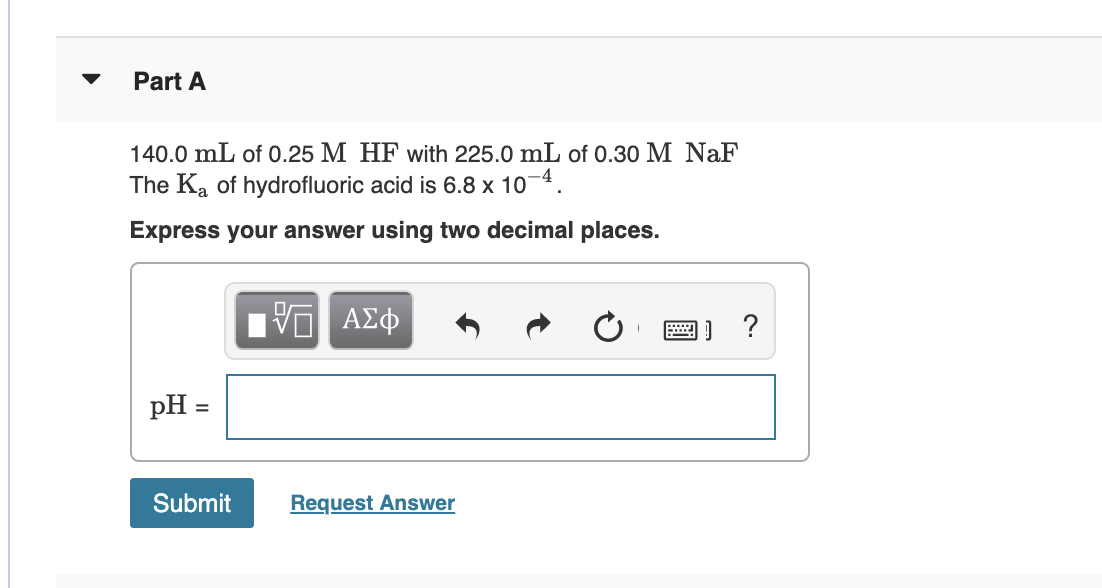
Transcribed Image Text:Part A
140.0 mL of 0.25 M HF with 225.0 mL of 0.30 M NaF
The Ka of hydrofluoric acid is 6.8 x 10¬4.
Express your answer using two decimal places.
ΑΣφ
pH =
Submit
Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- ITTL/ What is the pH of 92.9 mL of a solution which is 0.29 M in NaCN and 0.61 M in HCN? For HCN use K₂ = 4.9x10-10. Your Answer:arrow_forwardWhat is pOH of a solution consisting of 1.05 M NH3 and 0.98 M NH4NO3? Ka of NH4 + is 5.8 x 10-10 Group of answer choices 9.07 4.73 4.93 9.27arrow_forwardWhat is the pH when 20.0 mL of 0.10 M sodium hydroxide is added to 40.0 mL of 0.15 M HIO (Ka = 2.0 × 10–11) Group of answer choices 10.40 11.60 7.54 10.70arrow_forward
- Post-lab Question #2: What is the pH of the solution obtained by mixing 30.00 mL of 0.250 M HCI and 30.00 mL of 0.125 M NaOH? We assume additive volumes. 1.20 2.38 7.00 10.1arrow_forwardPart E Find the percent dissociation of a 0.150 M solution of a weak monoprotic acid having Ka = 0.14. Express your answer in percent to two significant figures. VE ΑΣΦ Submit ? Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remaining Part F Complete previous part(s) %arrow_forwardTyped and correct answer pleasearrow_forward
- - Meet l ? 1 8:39 AM O 28% a docs.google.com Q1//A solution of o.1 M of BX salt with pH=9.5, the HX is weak acid and BOH a strong base. Calculate the ionization constant for the weak acid 10-2 10-6 O 10-5 Q2// 1000 ml of o.1M hydrochloric acid were added to 1000 ml of o.1M ammonium hydroxide, ammonium chloride salt was formed. Calculate the pH for the salt * solution. Kb=1.8 ×10-5 10.02 O 3.98 5.28 + 20arrow_forwardFile Edit View History Bookmarks 000 b Answered: Calculate the x | M Inbox (463) - sydneyshe x P Everybody Loves Raymo x ← → Carrow_forward-9 Consider a solution that contain C-H5N and C5H5NH given Kb= 1.7 x 10-⁹ Calculate the ratio [CsHsN] [CsHsNH] if the solution has a PH= 10arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY