
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
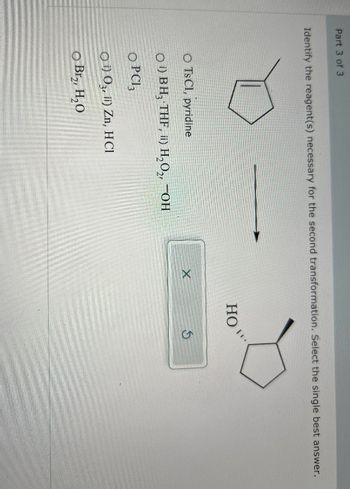
Transcribed Image Text:Part 3 of 3
Identify the reagent(s) necessary for the second transformation. Select the single best answer.
O Ts Cl, pyridine
O) BH, THF, ii) H₂O₂, OH
OPC3
O) O3, ii) Zn, HCl
O Br₂, H₂O
HO
X
S
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Given the following substitution reaction, what would the effect be of changing the solvent from CH3OH to (CH3)2S=O? CH₂(CH₂)4 CH₂-Br Select one: + Na* OH CH3(CH₂)4 CH₂-OH + Na Br A. The rate of the reaction would decrease. O B. The rate of the reaction would increase. O C. The rate of the reaction would not change.arrow_forwardi) Starting compounds Br HC=CH ii) Br CH3 "OH Br iii) a. the only productarrow_forwardFill in the reagents using the options in answer box.arrow_forward
- Q2. Find out all the unknown compounds (A, B and C) in the following multistep synthesis.arrow_forwardPropose reagent(s) or product(s) for the following transformation.arrow_forwardConsider the following multistep synthesis. KO Bu Br What is the identity of 3? CH3OH CH3CH₂OH 1 NaOCH3 HBr ROOR 2 3 More than one of these can accomplish this transformation. None of these can accomplish this transformation. Br₂ 4arrow_forward
- In both examples below the reactants shown are combined to bring about a nucleophilic substitution (S1, Sy2) and'or elimination (EI, E2) reaction What is the major reaction that takes place in each case? CH, CH, CH,CH,CHCCH, H20 Br NaNg CH,OH CH,CH,CHCH,CH, SN2arrow_forwardIdentify the best reagent to perform the transformation. H3C OH H3C CH3 Identify the best reagent. 1.H₂0+ 2. NaBr NaBr HBr (C6H5)3PBr₂ H3C H3C CH3 Brarrow_forwardThis is a multistep syntheses. More than one reagent/reaction will be necessary to produce the product. Provide all necessary reaction conditions (HBr, HgSO4, etc.) and the products produced after each step of the synthesis.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY