
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
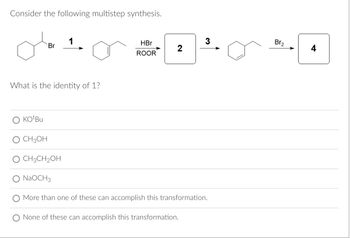
Transcribed Image Text:**Multistep Synthesis Problem**
Consider the following multistep synthesis:
1. A starting molecule has a bromine atom attached to a cyclohexane ring.
2. The first transformation (Step 1) involves converting this molecule into a cyclohexene.
3. In the second step, the cyclohexene is reacted with HBr in the presence of a peroxide (ROOR), resulting in a new intermediate.
4. This intermediate is then further reacted in step 3 with Br₂ to produce the final product.
**Question:**
What is the identity of reagent 1?
**Options:**
- ○ KOtBu
- ○ CH₃OH
- ○ CH₃CH₂OH
- ○ NaOCH₃
- ○ More than one of these can accomplish this transformation.
- ○ None of these can accomplish this transformation.
**Discussion/Explanation:**
The reagents provided in the options are potential bases or solvents that might facilitate the elimination or substitution reactions necessary for transformations. The identity of reagent 1 will determine the successful conversion of the starting bromocyclohexane into cyclohexene via an elimination reaction.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Given the following substitution reaction, what would the effect be of changing the solvent from CH3OH to (CH3)2S=O? CH₂(CH₂)4 CH₂-Br Select one: + Na* OH CH3(CH₂)4 CH₂-OH + Na Br A. The rate of the reaction would decrease. O B. The rate of the reaction would increase. O C. The rate of the reaction would not change.arrow_forwardWhat is the major product to the following elimination reaction? H20 Br hparrow_forwardIdentify the starting material necessary to carry out the following transformation. 1. NaOH, Br2 2. H3O* OH H OHarrow_forward
- 11 What is the missing reactant? CH3 Br LDA -78°C B THF 0 H-C-OCHS с i H-C-H -CH3 D CH3OHarrow_forwardWhat is the product of the following transformation? 1. HgSO4, H2SO4, H2O 2. НО OH Eto 3. LIAIH4 4. Нао HO ОН H HO HOarrow_forwardConsider the following multistep synthesis. KO Bu Br What is the identity of 3? CH3OH CH3CH₂OH 1 NaOCH3 HBr ROOR 2 3 More than one of these can accomplish this transformation. None of these can accomplish this transformation. Br₂ 4arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY