
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please provide answers in typed form and not a handwritten image.
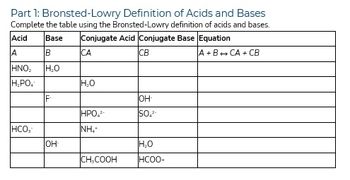
Transcribed Image Text:Part 1: Bronsted-Lowry Definition of Acids and Bases
Complete the table using the Bronsted-Lowry definition of acids and bases.
Base Conjugate Acid Conjugate Base Equation
Acid
A
B
ΤΗΝΟ,
H₂O
H₂PO4
HCO,
F
он-
CA
H₂O
HPO,²-
NH.
CH₂COOH
CB
OH-
SO,²-
H₂O
HCOO-
A+B+CA+CB
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 47 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Tyler the titrating student did not rinse his buret properly and residual water (not titrant) was left in the buret prior to filling the buret with base. how would the Precision, Accuracy, and Mass of CaCO3 in antacid be affected?arrow_forwardWeigh out accurately 9.99 g of copper(II) sulfate pentahydrate (CuSO4·5H2O) by taring using a clean and dry empty 250-ml beaker. 2. Add tap water to the beaker to reach the 50-mL mark on the side of the beaker. 3. Dissolve all the crystals of CuSO4·5H2O in the water using a clean glass rod for stirring. 4. Then using a glass funnel transfer all the blue solution to a 100-mL volumetric flask. Rinse the beaker with about 10 mL of water and transfer the solution to the volumetric flask. Repeat the rinsing of the beaker with another 10 mL of water and transfer the solution to the volumetric flask. This is called quantitative transfer of the solution. 5. Then add enough water to reach the calibration mark of the volumetric flask. (You will need to use a dropper to add the last few drops of water to ensure the meniscus is on the calibration mark). Close the volumetric flask with a stopper and mix the solution well (so that it is homogeneous). 6. You have now prepared a standard solution (or…arrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forward
- Question 4. In the course material you have been introduced to multiple charging for electrospray spectra. Each peak in the spectrum below can be used to provide a measure of the mass of this protein, you can work out the charge associated with each peak, and measures can be averaged to find the overall mass. Use simultaneous equations to find the charge associated with each peak in the spectra below, which will allow you to calculate the average mass of each species. Include an estimate of the error associated with this measure. 659.8 714.7 779.5 857,4 952.6 1071.5 BMW Sparrow_forward4) But the real angler continues, regardless of the local sage. What sort of tone does the author create by using the connotative effects of the word sage in paragraph 6? The word "sage" is used primarily as a joke, gently mocking the fishing guide who cannot force fish to A) appear or guarantee good fishing to a paying customer. Primarily, the author is creating None of awe or B) respect for the great wisdom and power that the Eiminate sage, or fishing guide, holds in his mind. The author is being highly critical, even condemning. of the ignorance of these so-called "sages who can't help fisherman catch fish. The word sage" in this context has no connotaive D) effect on the tone or meaning of this entire passage.arrow_forwardAluminum-lithium (AI-Li) alloys have been developed by the aircraft industry to reduce the weight and improve the performance of its aircraft. A commercial aircraft skin material having a density of 2.65 g/cm? is desired. Compute the concentration of Li (in wt%) that is required. The densities of aluminum and lithium are 2.71 and 0.534 g/cm3, respectively.arrow_forward
- Saved Normal BIIIU fxl Ix Actual mass used in solution prep (g) Volume solution prepared Measured conductivity (uS/cm) Substance 0.1 g Naci 0.1003 100 mL 1149 0.1 g Nal 0.10 100 ml 419 Calculate the mass of Nal that would be necessary to yield the same conductivity as the NaCl solution. Clearly show these calculations in your lab notebook. Saved T BII U X X + fr Normalarrow_forwardI am just not understand this problem at allarrow_forwardExplain if it is possible to combine different types of mass analyzer.arrow_forward
- Please show work, just like I need to put it in. Make clear HOW you got to that answer. Please write neat & make it readable. Again please write like I would need to put it in!arrow_forwardUnit 3-Two slides 11. Create a question that involves your compound and the formula m= n.M. Solve the question and show all of your work. wwwww 12. Create a question that involves your compound and the formula n = N/NA. Solve the question and show all of your work. TEarrow_forwardDiscuss three practical applications of solvent extraction in analytical chemistry.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY