
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
CAN YOU PLEASE WRITE THE ANSWER IN A WAY THAT IS EASY TO FILL IN THE CELLS PLEASE? I DO NOT UNDERSTAND WHAT IS WRITTEN HERE IN THE PREVIOUS BARTLEBY RESPONSE. THANK YOU!
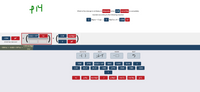
Transcribed Image Text:What is the change in enthalpy in kilojoules when 2.30 mol of Mg is completely
reacted according to the following reaction
2 Mg(s) + O2(g) →
2 MgO(s) AH = -1204
kJ
6.022 x 1023
kJ
2.30
mol MgO
-1204
X
STARTING AMOUNT
2.30 mol MgO
-1204 kJ x 6.022 × 1023 kJ ×
2 kJ
ADD FACTOR
DELETE
ANSWER
RESET
*( )
1000
2770
6.022 x 1023
5540
0.001
32.00
2
2.30
40.31
24.31
-1380
-2770
-5540
1380
-1204
1
kJ
g Mg
mol Mgo
J
g Mgo
mol O2
mol Mg
g O2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- I know you don't answer graded questions and I am not looking for you to straight up give me the answer to this but how do I even start? like I said before I know that the highest peak is 16amu which is Oxygen but do I just the vertical bar as a ratio? Like the next bar to the left (15amu?) is like 90% intensity? so like 9:10 ratio? There isn't even a 15amu element so please just give me some idea where to start. The place in the book it tells you to go to read has nothing about this stuff, its about finding empirical formulas from mass or percentages.... please help me!arrow_forwardHow much Mol S2O32- consumed and how much I3- was produced?arrow_forwardNo need to solve anything under the calculations. I've provided it so that the, Explanation question could be answered.arrow_forward
- No plagiarism Please! Station #1 = HNO2 (aq) For this acid, what does the white bead represent?________ blue bead? __________ 3rd Color Bead? _________ Record your observations in the particulate drawing. How many acid molecules have broken into ions? Determine the % ionization for the acid. Write the ionization reaction for this acid. ________ ↔ ______ + _______arrow_forwardx CHE101_02: Intro to General Che X Home 101 Chem101 My Questions | bartleby app.101edu.co myClackamas Login CUnofficial Transcri... W Logon Oregon Scholarsh... Home FAFSA on... Welcome to the ... The National Soci... Apps Submit Question 5 of 8 How many moles of lithium hydroxide would be required to produce 75.5 g of Li2COs in the following chemical reaction? 2 LIOH(s)+CO2 (g) --- LI2CO3 (s) + H2O (I) mol 1 2 с 7 +- 0 x 10 0 7:27 PM Type here to search ENG 11/13/2019 X CO LO 4tarrow_forwardA.) In the laboratory, a general chemistry student measured the pH of a 0.589 M aqueous solution of diethylamine, (C2H5)2NH to be 12.321.Use the information she obtained to determine the Kb for this base.Kb(experiment) =arrow_forward
- just 2 pleasearrow_forward1. Determine the Reaction Order for HCl using calculations described in the background section. Show your work. Note that your answer will probably not be a whole number as it is in the examples, so round to the nearest whole number. 2. Determine the Reaction Order for Na2S2O3 using calculations described in the Background section. Show your work. Note that your answer will probably not be a whole number as it is in the examples. 3. Write the rate law for the reaction between HCl and Na2S2O3. 4. Describe sources of error in this experiment?arrow_forwardA student used a pH meter to collect data for the titration of an unknown concentration of propanoic acid with a 0.150 mol/L solution of sodium hydroxide. The data table from the investigation is shown in the table below. Data for the Titration of Propanoic Acid with Sodium Hydroxide Volume of propanoic acid: 25.00 mL [NaOH] = 0.150 mol/L Volume of NaOH (aq) added (mL) 0.00 2.00 4.11 7.98 11.95 14.08 16.05 17.00 17.21 17.39 17.62 17.99 18.18 18.39 18.80 20.00 22.03 PH 2.83 3.84 4.20 4.64 5.03 5.27 5.61 5.90 6.00 6.09 6.23 6.74 8.80 10.92 12.24 12.56 12.69 Adobe 4343625416 00..arrow_forward
- S nomewUTK Answered Due Today, 11.3FM Fill in the Blanks Type your answers in all of the blanks and submit X, 2- s P F سه } AT T T & ce A €arrow_forwardplease give calculation as well. Calculate the pH value of each of the solutions in tubes 1-9 using the Henderson-Hasselbalch equation (H-H eqn).Determine the pl of casein. Compare your experimental values with those found in the literature. Biomolecules. Thank you! The protein is casein.arrow_forwardNo plagiarism Please! Station #8 = HCl (aq) For this acid, what does the white bead represent?________ blue bead? __________ 3rd Color Bead? _________ Record your observations in the particulate drawing. How many acid molecules have broken into ions? Determine the % ionization for the acid. Write the ionization reaction for this acid. ________ → ______ + _______arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY